Initial Evaluation of Thyroid Nodules
Clinical Outline
= Primers in Thyroidology
= Journal Club Presentation
= Case-based Discussion (inc. roundtables)
= Grand Rounds Lectureship
Management Recommendations
1. Clinical Background
A. Past Medical History1–3
ATA // AACE // JAES
In patients with confirmed or suspected thyroid nodules, the following personal and family history should be elicited:
- Family history for pheochromocytoma, hyperparathyroidism, and thyroid cancer in a first degree relative,
- Personal history of other malignancies,
- Childhood head and neck radiation therapy,
- Total body radiation for bone marrow transplant,
- Exposure to ionizing radiation,
- Symptoms of hoarseness, difficulty swallowing, and compression in the neck.
Supplemental Educational Content
It May Not All Be Overdiagnosis: The Potential Role of Environmental Exposures in the Thyroid Cancer Incidence Increase
Presenter: Maaike van Gerwen, MD
Summary
- 4:53 — Factors Affecting Incidence
Dr. van Gerwen discusses potential factors that can lead to increasing incidence of thyroid cancer, such as overdiagnosis, obesity, and heavy metal exposure. - 21:37 — Endocrine Disrupting Chemicals (EDC)
Dr. Van Gerwen explains how exposure to certain chemicals alters endocrine function and increases risk of thyroid cancer, using the aftereffects of the World Trade Center disaster as an example. - 37:02 — Proteomics, Metabolomics & EDCs
Dr. van Gerwen highlights another study she conducted where her team used metablomics and proteomics to study pathways linking EDCs with thyroid cancer.
Gender Inequity in Thyroid Cancer Diagnosis
Presenter: Karissa LeClair, MD
Summary
Dr. Karissa LeClair discusses the issue of gender inequity in thyroid cancer diagnosis. Dr. Louise Davies, Dr. Margaret Brandwein-Weber, Dr. Camilo Gonzalez-Velazquez, and Dr. Mack Harrell join us as panelists to drive discussion for this session.
- Thyroid cancer incidence has increased over time, but without a corresponding change in mortality. (4:59)
- A 2014 study showed that the absolute increase in thyroid cancer in women was almost 4 times greater than that of men. (8:23)
- There was no difference in the reservoir of latent PTC between men and women. In other words, the underlying prevalence of thyroid cancer was equal in men and women, which directly contrasted with incidence and mortality trends. (12:30)
- The diagnosis ratio between men and women stratified depending on the lethality of the thyroid cancer subtype. (15:38)
- Women are disproportionately diagnosed with small, localized papillary cancers (4.28:1). (17:09)
- Women are more likely to be diagnosed with subclinical thyroid cancer. Men are at risk of later diagnosis. (18:07)
- Women utilize healthcare more often than men. This generates more opportunities for surveillance and diagnosis. (18:37)
- Women are more likely to be referred for thyroid ultrasound, and are more likely to undergo thyroid ultrasound as initial imaging. These practice variations may contribute to greater detection of small, localized papillary cancers in women. (19:19)
- The disparate rates of thyroid cancer in women have been primarily driven by the detection of small, localized papillary cancers without significant mortality benefit. (21:30)
- The gender disparity seen in thyroid cancer diagnosis may be primarily driven by variations in healthcare utilization and our own practice patterns. (22:02)
- Discussion of potential geographic disparities in thyroid cancer incidence begins. (31:58)
- Discussion of the prevalence of Hashimoto’s thyroiditis among women begins. (39:11)
- Discussion of usage of the phrase “gender inequity” begins. (51:02)
Tables & Figures
Table 4. Familial & Heritable Forms of Thyroid Cancer
Familial Adenomatosis Polyposis
| Gene | APC |
| Benign Thyroid Disease | 40% |
| Cancer | 0.4–12% |
| Cancer Types | CMV-PTC: 63% FV-PTC: 25% PTC: 12% |
PTEN-Hamartoma Tumor (Cowden)
| Gene | PTEN |
| Benign Thyroid Disease | 75% |
| Cancer | 35% |
| Cancer Types | PTC: 50% FV-PTC: 28% FTC: 14% |
Carney Complex Type 1
| Gene | PRKARIA |
| Benign Thyroid Disease | ≤ 75% |
| Cancer | < 5% |
| Cancer Types | PTC FTC |
RET-Associated
| Gene | RET |
| Benign Thyroid Disease | — |
| Cancer | 100% |
| Cancer Types | MTC |
DICER1
| Gene | DICER1 |
| Benign Thyroid Disease | ≤ 30% |
| Cancer | — |
| Cancer Types | FTC FV-PTC |
CMV = Cribiform Morular Variant; FV = Follicular Variant
Percentages refer to disease prevalence.
B. Physical Exam1–3
ATA // AACE // JAES
In patients with risk factors for thyroid cancer or thyroid nodule related symptoms, a physical examination should be performed that focuses on the thyroid gland and adjacent cervical lymph nodes.
2. Laboratory Tests
A. Evaluation of TSH, TG & Calcitonin
ATA // ESMO // JAES
Measuring TSH is recommended to evaluate newly diagnosed thyroid nodules. Hyperfunctional nodules are more likely to be benign than non-hyperfunctional nodules.1,2
Serum thyroglobulin measurement before total thyroidectomy is not an accurate test to assess for thyroid malignancy. Therefore, in patients with thyroid nodules, serum thyroglobulin measurement is not recommended.1,2
Serum calcitonin may help identify patients with thyroid nodules harboring medullary thyroid cancers.4
- However, because medullary thyroid cancer is rare, and calcitonin levels could be falsely elevated, guidelines are discordant in regard to routine measurement of serum calcitonin in patients with thyroid nodules.1,4
3. Imaging Studies
A. Ultrasound (US) in Thyroid Nodule Assessment
ATA // NCCN // TIRADS
It is recommended to perform a thyroid ultrasound with cervical lymph node assessment in all patients suspected to have a thyroid nodule, or for whom a nodule has been previously identified through physical exam or incidentally on other imaging modalities.
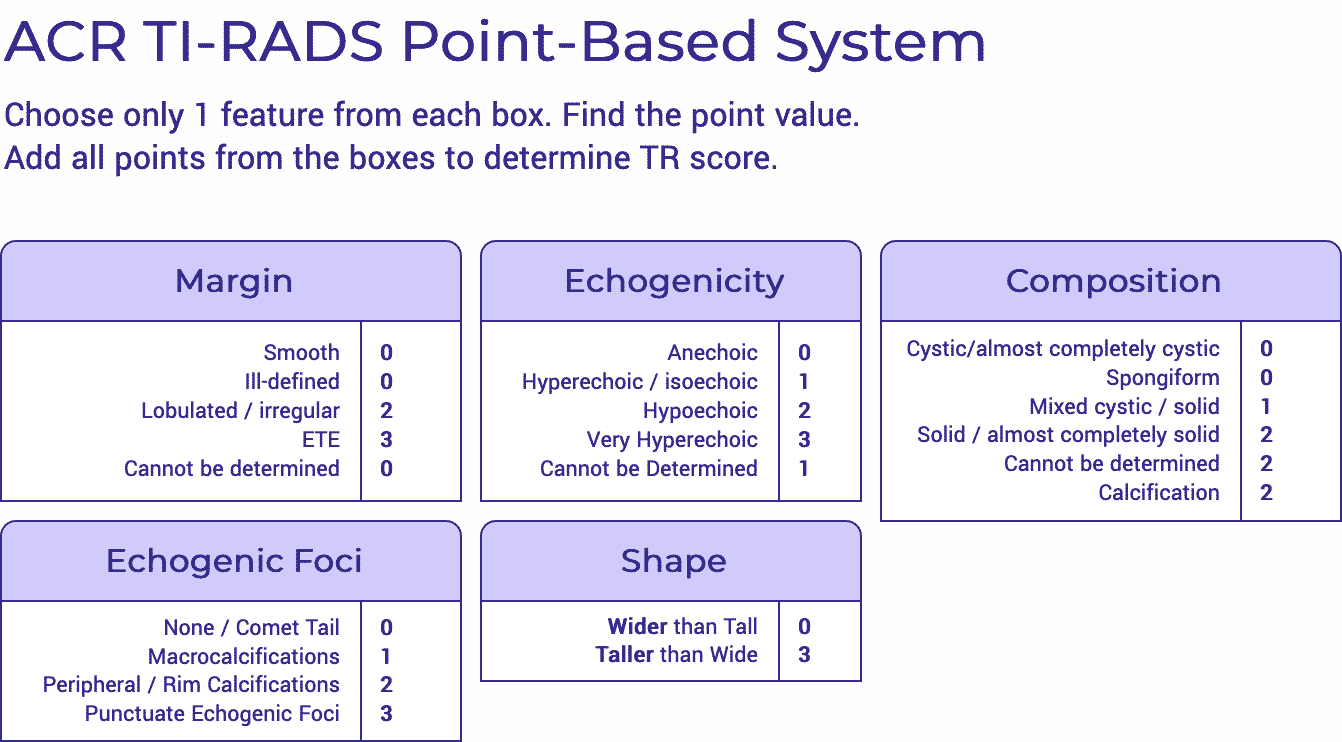
It is recommended to evaluate and record ultrasound characteristics such as composition, echogenicity, shape, characteristics of the nodule’s margins and presence of echogenic foci.1,5,6
It is recommended to assess the entirety of a nodule in order to describe characteristics including size, location, composition, echogenicity, margins, shape, and presence or absence of calcifications. These features help classify the risk of malignancy and guide FNA decision making.
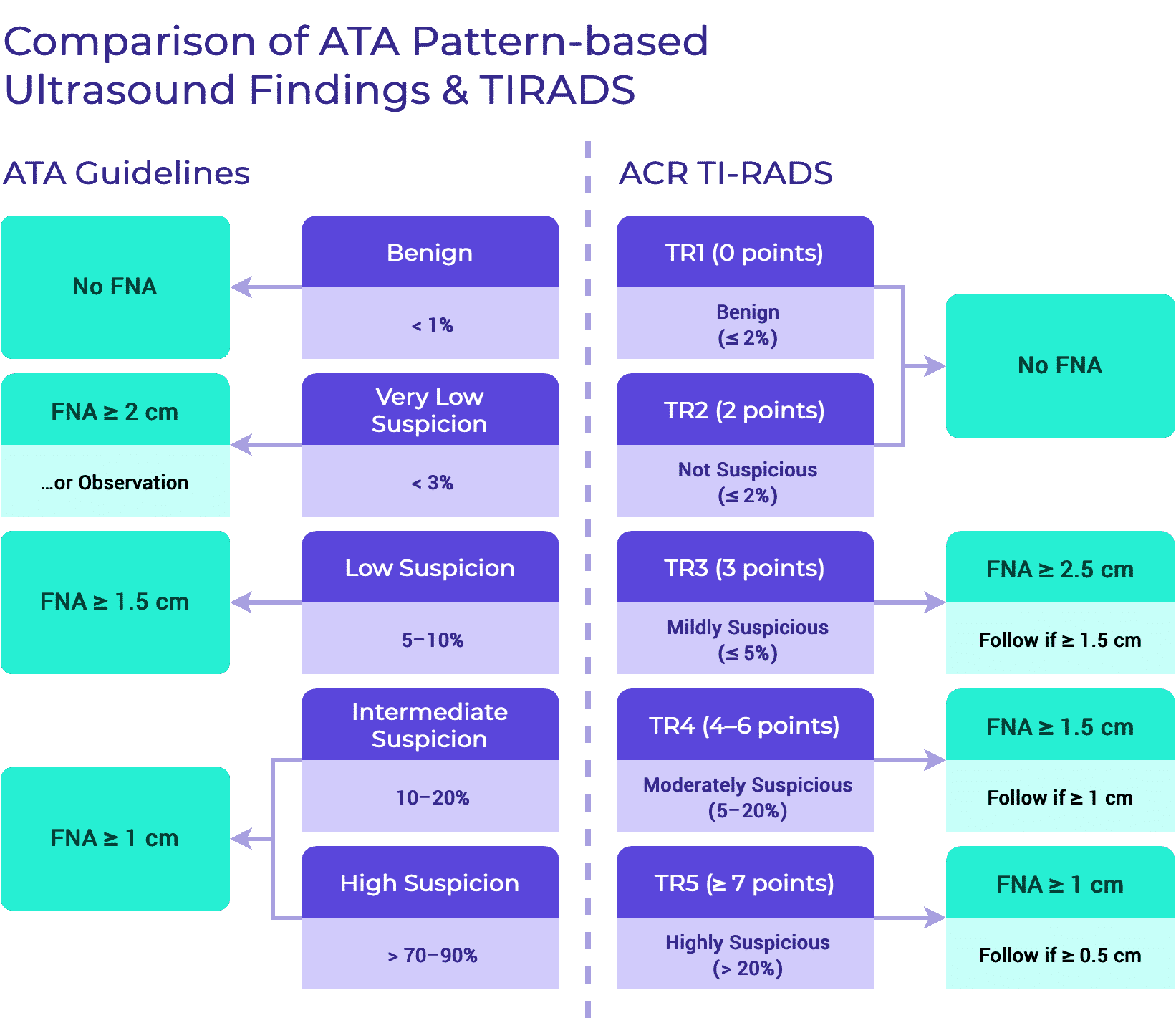
There is agreement between guidelines regarding the sonographic features that should be assessed. However, mild discordance exists in the use of classification systems used to aid FNA decision making. The two most commonly used classification systems are based on:1,5,6
- Ultrasound pattern recognition.
- Point-scoring systems based on sonographic features.
i. Sonographic Pattern-based Approach
ii. Scoring Systems Based on Individual Sonographic Characteristics
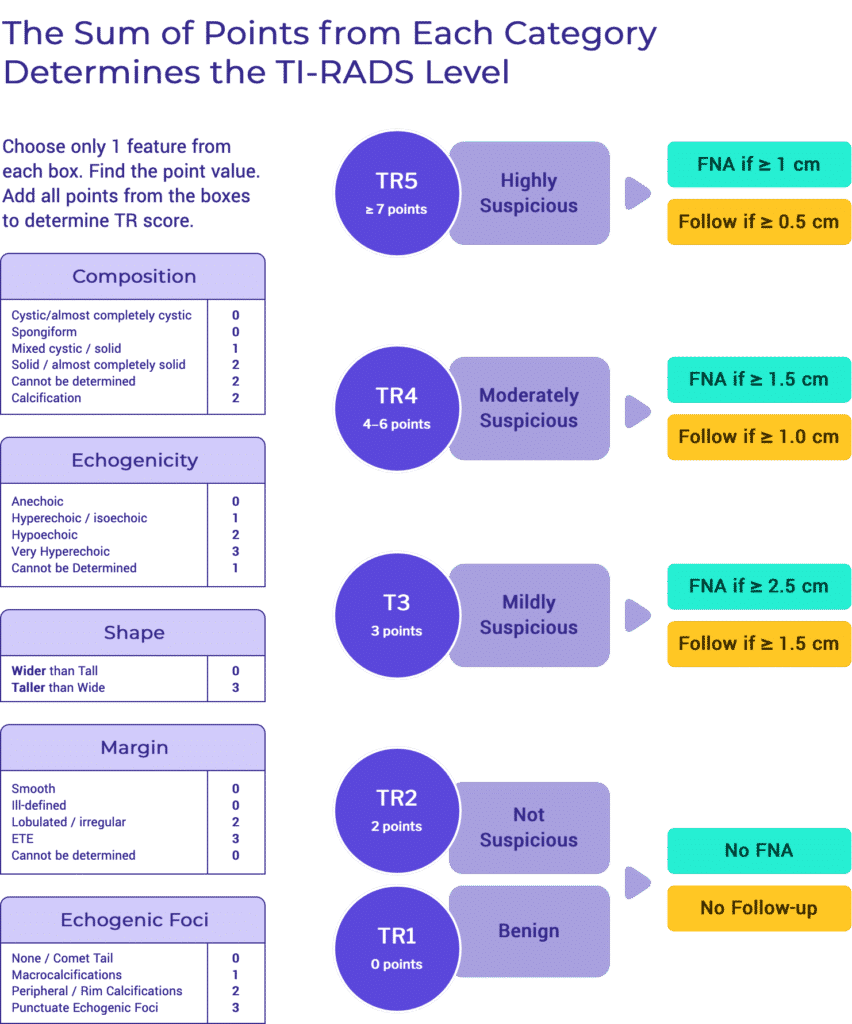
Recommendations for FNA biopsy are based on a nodule’s sonographic characteristics (TIRADS level) and the nodule’s maximum diameter. In levels TR3 to TR5, this system suggests size thresholds for which FNA is recommended, as well as lower size limits for which follow-up ultrasound examinations are recommended.6
B. US for FNA Decision Making1,5,6
ATA // NCCN // TIRADS
- Fine needle aspiration, with ultrasound guidance (UG-FNA), is the gold standard for diagnostic evaluation of clinically relevant thyroid nodules. Clinical relevance is determined by measurement of nodule size and analysis of the following sonographic features: microcalcifications, echogenicity, margins, cystic content, and shape.
- UG-FNA is not recommended when nodule diameter is < 1 cm regardless of sonographic features.
FNA Using Point-Based Approach
- Fine needle aspiration, with ultrasound guidance (UG-FNA), is the gold standard for diagnostic evaluation of clinically relevant thyroid nodules. Clinical relevance is determined by measurement of nodule size and analysis of the following sonographic features: microcalcifications, echogenicity, margins, cystic content, and shape.
- UG-FNA is not recommended when nodule diameter is < 1 cm regardless of sonographic features.
- It is recommended to perform UG-FNA on highly suspicious nodules > 1 cm in diameter. Highly suspicious nodules are defined as nodules with a sum of ≥ 7 points based on the presence of sonographic characteristics.
- Highly suspicious nodules may be observed with US if smaller than 1 cm.5,6
- It is recommended to perform UG-FNA on moderately suspicious nodules > 1.5 cm in diameter. Moderately suspicious nodules are defined as nodules with a sum in range of 4–6 points based on the presence of sonographic characteristics.
- Moderately suspicious nodules may be observed with US if smaller than 1.5 cm. 5,6
- It is recommended to perform UG-FNA on mildly suspicious thyroid nodules > 2.5 cm in diameter. Mildly suspicious nodules are defined as nodules with a sum of 3 points based on the presence of sonographic characteristics.
- Mildly suspicious nodules may be observed with US if smaller than 2.5 cm. 5,6
- UG-FNA is not recommended for nodules that are deemed to be not-suspicious based on their sonographic appearance. Thyroid nodules are classified as non-suspicious if their sonographic appearance results in a sum of 2 points.
- Thyroid nodules that are classified as not-suspicious do not require follow-up with US.
- UG-FNA is not recommended for nodules that are deemed to be benign based on their sonographic appearance. Thyroid nodules are classified as benign if their sonographic appearance results in the sum of 0 points.
- Benign appearing thyroid nodules do not require follow-up with US.5,6
FNA Using Pattern of Suspicion1
- It is recommended to perform UG-FNA on thyroid nodules > 1 cm in diameter with high suspicion sonographic pattern. Highly suspicious nodules are defined as hypoechoic nodules with one or more of the following features:
- Irregular margins, microcalcifications, taller than wide shape, rim calcifications with small extrusive soft tissue component or evidence of ETE.
- It is recommended to perform UG-FNA on thyroid nodules > 1 cm in diameter with intermediate suspicion sonographic pattern. Intermediate suspicious nodules are defined as hypoechoic nodules without microcalcifications, ETE or taller than wide shape.
- It is recommended to perform UG-FNA on thyroid nodules > 1.5 cm in diameter with low suspicion sonographic pattern. Low suspicious nodules are defined as hyperechoic or isoechoic nodules, or partially cystic nodules with eccentric solid areas without microcalcifications, irregular margins or ETE or taller than wide shape.
- UG-FNA may be considered on thyroid nodules > 2 cm in their greatest dimensión with very low suspicion sonographic pattern. Very low suspicious nodules are defined as spongiform or partially cystic nodules without any of the sonographic features described in the low, intermediate or high suspicion patterns.
- Purely cystic nodules and other nodules that do not meet the above described criteria are not suggested to undergo US-FNA biopsy.
Supplemental Educational Content
Investigating the Effect of Thyroid Nodule Location on the Risk of Thyroid Cancer
Presenter: Sina Jasim, MD, MPH & Franklin N. Tessler, MD
Summary
Dr. Sina Jasim and Dr. Franklin Neil Tessler discuss study findings related to the impact of thyroid nodule location on the risk of thyroid cancer and comment on the implications that these findings have for radiological risk stratification systems.
- Dr. Sina Jasim provides an overview of both intrinsic and nonintrinsic features that determine nodule sampling. Intrinsic features include ultrasound features and contrast-enhanced sonography, while noninstrinsic features consist of lymph node involvement, extra-thyroidal invasion and perhaps location of a nodule.
- In an overview of her study investigating the effect of thyroid nodule location on the risk of thyroid cancer, Dr. Sina Jasim concludes that location is an independent risk factor for risk of malignancy and that such risk of malignancy is higher in the isthmus region.
- Dr. Sina Jasim describes the performance of revised risk algorithms that reflect added risk for thyroid nodules in particular geographic regions. She highlights a decrease in performance for all revised algorithms except an addition of 1 point to isthmus nodules.
- Dr. Franklin Tessler emphasizes issues associated with the large number of thyroid imaging risk systems and guidelines, which led to his formation of the International Thyroid Ultrasound Working Group.
Macrocalcifications & Malignancy Risk Within the ATA Sonographic Pattern System
Presenter: Kristen Kobaly, MD
Summary
The American Thyroid Association Sonographic Pattern System (ATASPS) depicts 5 levels of suspicion for malignancy based on the sonographic appearance of a thyroid nodule. However, 3–37% of nodules are non-classifiable when the combination of grayscale findings is not depicted by the ATASPS.
The only calcifications included in the ATASPS are in solid hypoechoic high suspicion (HS) nodules and include both microcalcifications and peripheral interrupted calcifications with soft tissue extrusion. Non-hypoechoic nodules with these and other calcification patterns, which we defined as non-high suspicion calcifications (NHSC), are not classifiable by ATASPS. We assessed the effect of assigning an ATASPS risk level to nodules with NHSC based on analysis of their other grayscale features.
- 7:47 Thyroid Nodule Calcifications in the Literature
Dr. Kristen Kobaly discusses trends in the literature surrounding thyroid nodule calcifications, emphasizing the heterogeneity in studies which analyze macrocalcifications. - 25:42 Macrocalcifications and the Future
Dr. Kobaly proposes recommendations for evaluating the level of risk associated with macrocalcifications in thyroid nodules moving forward based on findings from her study. - 45:51 Why Analysis of Macrocalcifications Matters
Dr. Javad Azadi describes the importance of analyzing the risk associated with macrocalcifications in thyroid nodules, highlighting significant discrepancies between ACR-TIRADS and ATASPS with regard to this feature.
Clinician Agreement on the Classification of Thyroid Nodules Ultrasound Features: A Survey of Two Endocrine Societies
Presenter: Nydia Burgos, MD
Summary
- 9:20 Review of Results
Dr. Burgos presents a series of graphs displaying the level of inter-rater agreement as compared to the benchmark AC1 value of 0.6 that defines the boundary between moderate and substantial agreement. - 16:31 Dr. Tessler’s Presentation
- 19:34 ACR TI-RADS
Dr. Tessler presents the ACR TI-RADS 5 feature categories and 18 descriptors
4. Management of Benign Thyroid Nodules1
ATA
Surgery may be considered for benign thyroid nodules when > 4 cm in diameter, especially if a pattern of growth is detected or if there are compressive symptoms.
5. Molecular Testing1
ATA // NCCN
- Molecular testing may help guide clinical management in patients having an indeterminate thyroid FNA result (Bethesda III or IV) without a high level of clinical or radiologic concern for malignancy.5
- Molecular testing may also be considered for patients with a suspicious for malignancy (Bethesda V) thyroid FNA result if the results of such testing would impact surgical management.
- Such molecular testing should be performed in appropriately certified laboratories (CLIA or CAP in the US).
- Patients should be counselled regarding the benefits and limitations of molecular testing and the uncertainty regarding the significance of molecular testing results.
- Although not explicitly stated by ATA guidelines, there is concordance that if molecular testing results (in conjunction with clinical and radiologic findings) suggest a benign lesion, nodule surveillance as per guidelines for a benign FNA result is recommended.5
- If molecular testing is suggestive of malignancy, diagnostic excision (lobectomy or total thyroidectomy) is recommended.5
- If molecular testing indicates a high likelihood of papillary carcinoma (particularly a BRAFV600E mutation, RET/PTC translocation), management should be as per guidelines for a diagnosis of papillary carcinoma by FNA.
Related: Initial Thyroid Cancer Management
- No specific molecular test is endorsed as being preferred.
Supplemental Educational Content
Predictive Value of a Genomic Classifier in Indeterminate Thyroid Nodules Based on Nodule Size
Presenter: Babak Givi, MD
Importance: Genomic classifiers were developed to better guide clinicians in the treatment of indeterminate thyroid nodules (ITNs). To our knowledge, whether there is variation in the diagnostic accuracy of these tests depending on ITN size has not been previously studied.
Objective: To analyze the diagnostic performance of a genomic classifier in relation to ITN size.
- 22:23 Genomic Profiles of Malignant Nodules
Dr. Givi presents a genomic profile of cancerous nodules and their genetic mutations. - 28:19 Poorly Differentiated Thyroid Mass
Dr. Givi presents a case in which Thyroseq identified no thyroid-specific genetic alterations. - 40:20 NIFTP
Dr. Nikiforov discusses NIFTP (non-invasive follicular thyroid neoplasm with papillary-like nuclear features) that must be surgically resected.
Performance of a Multigene Genomic Classifier in Thyroid Nodules With Indeterminate Cytology
Presenter: Yuri Nikiforov, MD, PhD
Dr. Yuri Nikiforov presents a prospective double-blind multicenter international study carried out to determine the clinical validation of ThyroSeq v3 GC. Dr. Zubair Baloch joins as a featured guest discussant and presents on the current status of thyroid cytology.
- Fine needle aspiration biopsy efficiently determines the diagnostics of thyroid nodules. However, 20-30% of nodules are categorized as indeterminate. Molecular testing is utilized to identify whether these nodules are benign or malignant, and whether they are low risk or high risk, which is important information used to decide patient management.
- Dr. Yuri conceptualizes the sensitivity and specificity of the Multigene Genomic Classifier in a sample size of 100 patients with Bethesda III-IV cytology (17:20).
- Dr. Yuri calls attention to the fundamental issue of using a binary classification system for nodules (either benign or malignant), when in reality there is a continuum of nuclear features. This challenge led to the introduction of the concept “NIFTP” (Non-Invasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features).
- Dr. Baloch highlights the inclusion of indeterminate categories in the 1st edition Bethesda Classification Scheme for thyroid nodule cytology. The application of this classification scheme revealed the variance in risk of malignancy, especially for the indeterminate categories. This finding led to the creation of the second edition of this system.
Genetic Mutations in Thyroid Cancer
Presenter: Jeffrey Krane, MD, PhD
Dr. Jeffrey Krane gives a primer lecture on genetic mutations in thyroid cancer management and their role in diagnosis and treatment.
- The Cancer Gene Atlas (TCGA) Project revealed a variety of previously undiscovered driver mutations of papillary thyroid carcinoma (PTC).
- TCGA allowed us to separate papillary carcinomas into 2 broad groups of tumors: BRAF-like (tall cell variants and classic) and RAS-like (FVPTC, resemble follicular neoplasms).
- The molecular landscape of thyroid neoplasms is complex.
- The mere presence of a mutation does not necessarily equate with the presence of malignancy, particularly in the RAS family of mutations.
- There are certain high-risk mutations for which mere identification is likely to portend the presence of a malignancy (e.g. BRAF V600E, TERT promoter).
- There are also intermediate-risk mutations (e.g. RAS).
- Generally speaking, it is not necessary to know the underlying mutational profile of a thyroid tumor in order to render a diagnosis, as most diagnoses are based on morphology or phenotype.
- However, molecular testing is important in the context of cytology, especially for low-risk indeterminate aspirates (Bethesda III and IV).
- Molecular testing can be used as an intervention to help determine whether a patient will require surgery, the extent of surgery required, or appropriate targeted therapies for advanced disease.
- Commercially popular tests include Thyroseq v3 (UPMC/PathCBL), Afirma GSC (Veracyte), and ThyGeNEXT/ThyroMIR (Interpace Diagnostics).
Molecular Diagnostics 101
Presenter: Jane Houldsworth, PhD
Drs. Houldsworth and Mehrota provide a higher-level insight into the basics of the assays and technologies used to detect somatic genetic mutations, translocations, and copy number alterations.
Summary
At this week’s Virtual Journal Club, we were excited to welcome Dr. Jane Houldsworth and Dr. Meenakshi Mehrota. Dr. Houldsworth is the Director of the Molecular Clinical Laboratory and Vice Chair of the Molecular Pathology Division at Mount Sinai. Dr. Mehrota is an Assistant Professor of Pathology at Mount Sinai, and also serves as the Assistant Director of the Molecular Pathology Laboratory. Together, they offered a comprehensive overview of the assays and technologies used to detect somatic mutations and identify genetic translocations and copy number alterations. Their research has significantly contributed to the translation research at Mount Sinai in an effort to apply new diagnostic platforms to clinical oncology.
Dr. Houldsworth discussed the basics of tumor burden, which is calculated as the number of tumor nuclei ÷ (number of tumor nuclei + number of benign nuclei).
- The detection and sensitivity of tumor burden analysis critically informs the method of tumor dissection.
- The higher the tumor burden, the higher the higher the likelihood of detecting the mutation that is driving the cancer formation
When analyzing the patient’s genetic information, there are varying levels of specificity that range from comprehensive all the way to single gene sequencing.
- Whole Genome Sequencing (WGS): the method of sequencing everything within the chromosome in a nucleus. This includes the exons that become the final transcript for protein information and the introns that are removed prior to protein encoding.
- Whole exome sequencing (WES): methods of sequencing just the exons of the protein-coding genes.
- Target Sequencing: Allows analysts to select for regional hotspots where mutations are frequently found.
- We can now even get as specific as single genes and single variants within those genes! The more specific the analysis, the less comprehensive but much better chance of detection.
Dr. Mehrota then enlightened us on the basic details of polymerase chain reaction (PCR). PCR is a method of replicating a specific DNA sequence in mass quantities to allow for a more detailed analysis. Sanger’s Sequencing, for example, utilizes PCR
Effectiveness of Molecular Testing Techniques for Diagnosis of Indeterminate Thyroid Nodules: A Randomized Clinical Trial
Presenter: Masha Livhits, MD
Summary
Dr. Masha Livhitz and Dr. Bryan McIver discuss the role of molecular marker testing in reference to diagnosis of indeterminate thyroid nodules.
Dr. Masha Livhits presents the dilemma of the indeterminate thyroid nodule and the role that molecular marker testing can play in addressing this dilemma.
Dr. Masha Livhits discusses findings from a study which compared molecular marker tests (Afirma GEC and Thyroseq v2 in Phase 1, Afirma GSC and Thyroseq v3 in Phase 2).
- In Phase 1, Thyroseq v2 had better benign call rate and specificity as compared to Afirma GEC.
- In Phase 2, Thyroseq v3 and Afirma GSC performed equally well.
Dr. Bryan McIver highlights that molecular marker testing is moving away from use as a binary construct in which patients with any mutations receive total thyroidectomy and those without mutations are followed. Rather, specific types of mutations are assessed on a spectrum to determine the aggressiveness of treatment needed.
- For example, patients with low-risk mutations may receive therapeutic lobectomy rather than total thyroidectomy.
Performance of a Multigene Genomic Classifier in Thyroid Nodules with Suspicious for Malignancy Cytology
Presenter: John Skaugen, MD
Summary
- 6:46 — Evaluating Molecular Testing for Thyroid Nodules
Dr. Skaugen discusses his study goal, which is to evaluate the diagnostic performance of molecular testing (MT) for thyroid nodules that are suspicious for malignancy (SFM) in cytology. - 7:14 — Applying Molecular Testing to FNA Samples
Dr. Skaugen provides an overview of study methodology, discussing how MT was applied and how discrepant MT and histologic outcomes were resolved. - 11:01 — Malignancies & Mutations
Dr. Skaugen discusses how malignancy and mutation rates differed based on MT results. - 17:55 — Study Conclusions
Dr. Skaugen sums up study conclusions, emphasizing how MT can predict histologic outcome and molecular risk groups (MRGs) correlate with cancer recurrence risk in thyroid nodules with SFM cytology.
6. Management Based on Cytological Findings
ATA // NCCN // TIRADS
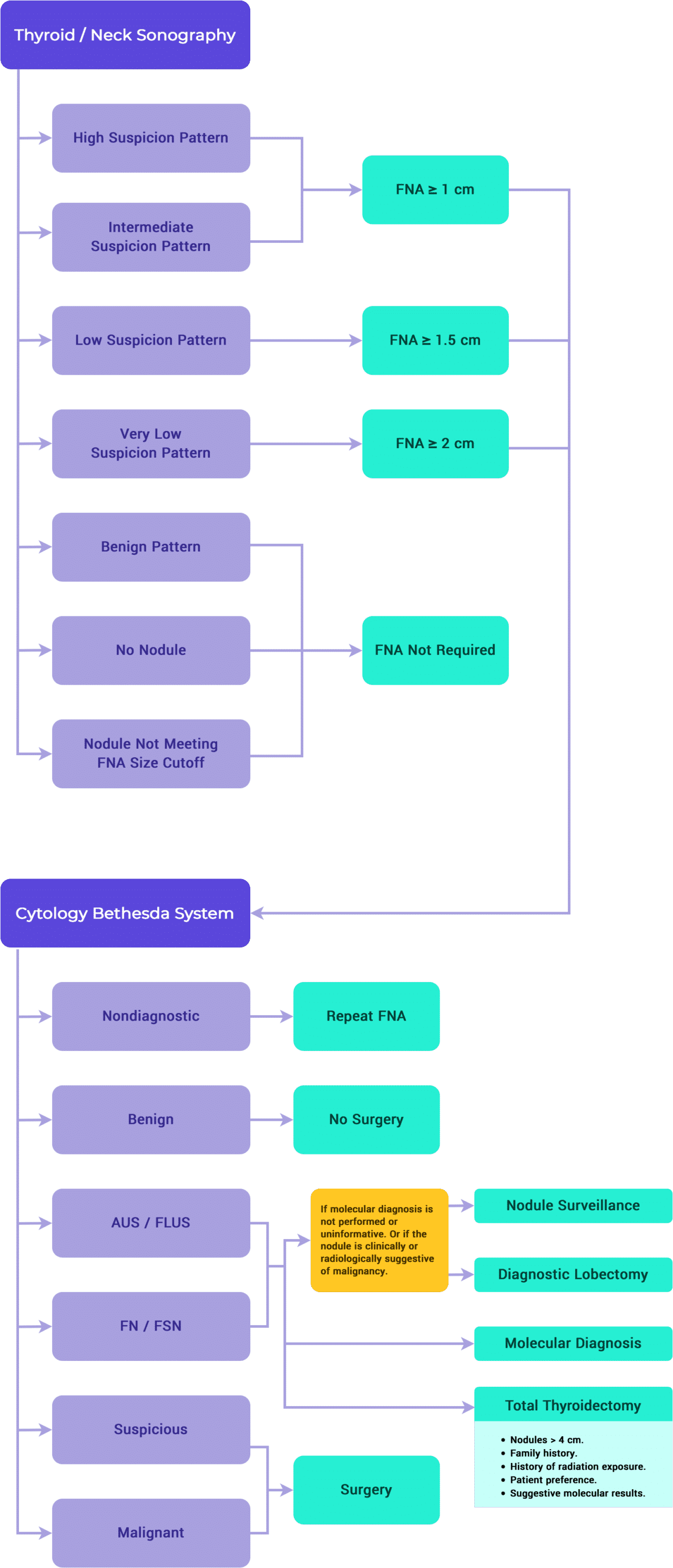
A. Non-diagnostic (Bethesda I)
| Risk of Malignancy |
| 5–10% |
| Included Histology |
| • Nondiagnostic or Unsatisfactory Cyst fluid only, • Virtually acellular specimen, • Other (obscuring blood, clotting artifact, drying artifact, etc). |
- An initial non-diagnostic result of a solid nodule should prompt repeat FNA with ultrasound guidance and on-site cytology evaluation if available.1,5
- If cystic, repeat FNA should be performed if any sonographically concerning areas are identified, Repeatedly non-diagnostic nodules that are not sonographically suspicious may be treated with continued surveillance or diagnostic excision.1,5
- Surgical excision should be considered if: 1,6
- The nodule is sonographically highly suspicious,
- Growth of > 20% in 2 dimensions is identified, or
- There is high clinical concern for malignancy.
B. Benign (Bethesda II)
| Risk of Malignancy |
| 0–3% |
| Included Histology |
| • Benign, • Consistent with a benign follicular nodule (includes adenomatoid nodule, colloid nodule, etc), • Consistent with chronic lymphocytic (Hashimoto) thyroiditis in the proper clinical context, • Consistent with granulomatous (subacute) thyroiditis, • Other |
No immediate treatment is needed for a benign FNA.1
Nodule surveillance should be performed in accordance with sonographic imaging risk stratification, as defined by:1,5,6
- High suspicion: repeat ultrasound and FNA biopsy within 12 months.
- Low-intermediate suspicion: repeat ultrasound at 12–24 months and consider repeat FNA for growth of > 20% in 2 dimensions (at least 2 mm or > 50% volume increase) or new concerning imaging findings.
- Very low suspicion: no surveillance or imaging after 24 months.
- If 2 benign FNA results are obtained on the same nodule, no further surveillance is needed for that nodule.
C. AUS/FLUS (Bethesda III)1,5
| Risk of Malignancy |
| 10–30% |
| Included Histology |
| • Atypia of Undetermined Significance • Follicular Lesion of Undetermined Significance |
Diagnostic lobectomy or nodule surveillance (guided by clinical and radiologic risk factors and patient preference) may be considered after an initial or repeat AUS/FLUS diagnosis, or if molecular testing is not performed or is uninformative.
Diagnostic lobectomy or total thyroidectomy is indicated if molecular testing yields a suspicious result.
Total thyroidectomy may be appropriate for diagnostic excision depending on imaging and clinical concerns, such as:
- Large nodule size (> 4 cm),
- Family history,
- History of radiation exposure,
- Patient preference, or
- Molecular testing results.
If molecular testing results (in conjunction with clinical and radiologic findings) suggest a benign lesion, nodule surveillance as per guidelines for a benign FNA result is recommended.
D. Suspicious for a Follicular (or Hürthle Cell) Neoplasm (Bethesda IV)1,5
| Risk of Malignancy |
| 25–40% |
| Included Histology |
| • Follicular Neoplasm, or • Suspicious for a Follicular Neoplasma, including Oncocytic (Hürthle cell) type |
If clinically or radiographically concerning for malignancy, diagnostic lobectomy or total thyroidectomy should be performed.
Otherwise, molecular testing may be considered to inform further decision making.
Diagnostic excision is indicated if molecular testing is not performed, is uninformative, or yields a suspicious result.
Nodule surveillance may be considered if the nodule is low-risk or there is patient preference for surveillance.
Total thyroidectomy may be appropriate for diagnostic excision depending on imaging and clinical concerns, such as:
- large nodule size (> 4 cm),
- family history,
- history of radiation exposure,
- patient preference, or
- molecular testing results.
If molecular testing results (in conjunction with clinical and radiologic findings) suggest a benign lesion, nodule surveillance as per guidelines for a benign FNA result is recommended.
Supplemental Educational Content
The Presence of Hürthle Cells Does Not Increase the Risk of Malignancy in Most Bethesda Categories in Thyroid Fine-Needle Aspirates
Presenter: Carrie Lubitz, MD, MPH
Dr. Carrie Lubitz presents a retrospective study to analyze how the presence of Hürthle cells affects risk of malignancy. Dr. Jeffrey Krane joins as a featured guest discussant and focuses on the cytopathologist’s perspective on Hürthle cells.
- The fourth edition WHO classification dedicated a distinct category to Hürthle Cell carcinoma because of its unique genetic profile
- This study concluded that the risk of malignancy, based on the 2017 Bethesda System for Reporting Thyroid Cytopathology, is not increased by the presence of Hürthle cells
- Dr. Krane details the non-neoplastic and neoplastic conditions Hürthle cells are seen in (20:34)
- Dr. Jeffrey Krane highlights that pathologists have different criteria for diagnosing Hürthle cells as well as for when it is necessary to include these findings in their reports (34:36)
- Dr. Lubitz and Dr. Krane comment on how size of a Hürthle cell lesion and the presence of TERT or RAS mutations impact interpretation (48:21)
E. Suspicious for Malignancy (Bethesda V)
| Risk of Malignancy |
| 50–75% |
| Included Histology |
| • Suspicious for Malignancy, • Suspicious for papillary thyroid carcinoma, • Suspicious for medullary thyroid carcinoma, • Suspicious for metastatic carcinoma, • Suspicious for lymphoma, • Other |
- FNA diagnosis of suspicious for malignancy should be managed similarly to a malignant cytologic diagnosis.1,5
- Molecular mutational testing may be considered for patients with a suspicious for malignancy (Bethesda V) thyroid FNA result if the results of such testing would impact surgical management.1
F. Malignant (Bethesda VI)1
| Risk of Malignancy |
| 97–99% |
| Included Histology |
| • Malignant, • Papillary thyroid carcinoma, • Poorly differentiated carcinoma, • Medullary thyroid carcinoma, • Undifferentiated (anaplastic) carcinoma, • Squamous cell carcinoma, • Carcinoma with mixed features (specify), • Metastatic malignancy, • Non-Hodgkin lymphoma, • Other |
- Surgery is generally indicated for a diagnosis of papillary carcinoma in nodules ≥ 1 cm.5
- Lobectomy may be appropriate for low-risk papillary carcinomas between 1–4 cm with:5
- No evidence of distant or nodal metastasis.
- No evidence of extrathyroidal extension.
- No prior radiation exposure.
- Otherwise, total thyroidectomy is preferred.5
- Surgery may not be indicated if:
- Patient has a short life expectancy.
- Patient has significant comorbidities increasing surgical risk.
- Patient has other urgent health concerns.
- Surgery is generally indicated for a diagnosis of medullary carcinoma regardless of size.
- Anaplastic carcinoma needs further evaluation to assess tumor resectability.5
7. Thyroid Nodule Evaluation During Pregnancy1
ATA
- FNA of clinically relevant thyroid nodules should be performed in euthyroid and hypothyroid pregnant women.7
- For women with suppressed serum TSH levels that persist beyond 16 weeks gestation, FNA may be deferred until after pregnancy and cessation of lactation. At that time, a radionuclide scan can be performed to evaluate nodule function if the serum TSH remains suppressed.
- PTC discovered by cytology in early pregnancy should be monitored sonographically. If it grows substantially before 24–26 weeks gestation, or if the US reveals cervical lymph nodes that are suspicious for metastatic disease, surgery should be considered during pregnancy. However, if the disease remains stable by midgestation, or if it is diagnosed in the second half of pregnancy, surgery may be deferred until after delivery.
Supplemental Educational Content
Thyroid nodule assessment & management during pregnancy
Presenter: Spyridoula Maraka, MD
Review of Epidemiology
Although the incidence of thyroid cancer is increasing around the world, thyroid cancer during pregnancy has received less attention in the literature
Review of Biology
- Low TSH may be related to pregnancy and may not be cause for concern
- Increase in thyroid volume may be related to changes in calcitonin and growth factors, in addition to iodine deficiency
- During pregnancy, daily iodine needs increase by 50%
- 150 mcg of iodine per day in the form of potassium iodine is highly recommended
Discussion of Evaluation
- Clinical history, neck palpation, blood test (thyroid function tests) neck ultrasound, FNA
- Thyroid scintigraphy and uptake not possible in pregnant women
- ATA guidelines recommend against LT4 suppressive therapy due to side of effect of thyrotoxicosis
- For a woman with benign nodules but compressive symptoms, sclerotherapy may be an option
- For a woman with indeterminate nodules, molecular testing has not been validated in pregnancy, and gestation may affect gene expression
- 1st and 3rd trimesters are not safe for surgery
- Studies have demonstrated equal outcomes for pregnant women who undergo surgery before or after delivery, mostly low-risk thyroid cancer
Review of Evidence for Management of Pregnant Women
What is the prevalence of thyroid nodules in pregnancy?
In two studies (Glinoer 1991 and Kung 2002), pregnancy was not a risk factor for thyroid nodule growth or malignant transformation
- The total thyroid volume remained stable (Glinoer 1991)
- Increase in volume but returned to baseline (Kung 2002)
In one study, researchers found that nodules do not increase in size during pregnancy
- Thyroid gland sizes returned to normal, gland size related to weight gain (Vannucchi 2017)
Is the frequency of thyroid cancer increased in pregnant women with thyroid nodules?
Still an ongoing question.
- Three retrospective studies of frequency of thyroid cancer: 12% (n=57), 15% (n=40), 43% (n=16), but these may be overrepresentations due to selection bias
- Kung 2002: 0% (n=34)
- Smith 2003: Prevalence was 14.4/100,000 live births within 1 year postpartum
Thyroid Cancer During Pregnancy
Presenter: Fabian Pitoia, MD
Dr. Fabian Pitoia discusses his approach to discussion with a new patient in the case of a thyroid cancer diagnosis during pregnancy.
Summary
- Dr. Fabian Pitoia describes the importance of calming a patient and her family down in the case of a thyroid cancer diagnosis during pregnancy.
- Dr. Fabian Pitoia asserts that there is likely not a worse outcome for thyroid cancer during pregnancy in comparison to thyroid cancer outside of this time.
Diagnostic & Treatment Considerations for Thyroid Cancer in Women of Reproductive Age and the Perinatal Period
Presenter: Evert van Velsen, MD
Summary
- 10:50 — Pregnancy & Thyroid Physiology
Dr. van Velsen discusses changes in thyroid size and serum thyroglobulin levels during pregnancy which normalize postpartum, as well as the frequency of thyroid nodules detected on imaging during pregnancy. He presents guidelines for treatment of differentiated thyroid cancer depending on pregnancy trimester. - 15:17 — More Pregnancy Guidelines
Dr. van Velsen discusses ATA guidelines regarding radioiodine therapy when pregnant or breastfeeding. Additionally, he presents conflicting evidence regarding the frequency of adverse pregnancy outcomes following radioiodine therapy. - 21:44 — Recommendations for Levothyroxine
Dr. van Velsen presents evidence from Alexander et al. (2004) and Korevaar et al. (2017) suggesting that levothyroxine dose should be increased by 25–30% when pregnant, though he cautions the risks of overtreatment. Lower serum TSH and higher free thyroxine (FT4) during pregnancy has been associated with impeded fetal development. - 33:47 — Dr. Davies Presents
Dr. Davies discusses the counterintuitive nature of how thyroid cancer does not seem to be any more dangerous during pregnancy than otherwise, despite basic science literature suggesting otherwise. He discusses various growth factors, specifically IGF-1, which stimulates thyroid cell growth by synergizing with TSH. This suggests that pregnancy should be a dangerous time for thyroid cancer, however it is not! - 40:52 — Dr. Davies’ Conclusions
Dr. Davies suggests that if the data is correct (given the small sample sizes), pregnancy physiology must somehow inhibit the consequences of thyroid cancer stimulation and growth. - 43:27 — Discussion with Q&A
Drs. Elise Brett and Mark Urken join the discussion. Dr. Brett asks why lactation should stop 6-weeks prior to undergoing radioiodine therapy as well as inquires about a trimester-based risk model for replacement of TSH.
8. Thyroid Nodule Evaluation During Childhood1
ATA
Children with DTC should be cared for by teams of physicians experienced in the management of DTC in children. This will facilitate interdisciplinary decisions regarding optimal therapy and will help to reduce the possibility that treatment and long-term follow-up will be either overly aggressive or inadequate
The evaluation and treatment of thyroid nodules in children should be the same as in adults with the exceptions that:
- US characteristics and clinical context should be used, rather than size alone, to identify nodules that warrant FNA,
- All FNAs in children should be performed under US guidance,
- Preoperative FNA of a hyperfunctioning nodule in a child is not warranted as long as the lesion is removed,
- A diffusely infiltrative form of PTC may occur in children and should be considered in a clinically suspicious gland, and
- Surgery (lobectomy plus isthmusectomy) is favored over repeat FNA for most nodules with indeterminate cytology.
A positive mutational test appears highly likely to be associated with malignancy. Conversely, insufficient data exist in children to rely on negative genetic studies to reliably exclude malignancy. Although molecular studies hold promise for complementing the results of FNA, particularly for nodules that yield indeterminate cytology, they have not yet been sufficiently validated in children and cannot be recommended in routine clinical practice until further studies are conducted.
Supplemental Educational Content
Differences in Adult & Pediatric Thyroid Nodule Cytology
Presenter: Ari Wassner, MD
Summary
Dr. Ari Wassner and Dr. Andrew Bauer elaborate on the differences between adult and pediatric thyroid nodule management and suggest techniques to improve the predictive precision when determining nodule malignancy in children.
- Dr. Ari Wassner presents an article that discusses the differences in thyroid nodule cytology and malignancy risk between children and adults.
- The point of thyroid nodule evaluation is to detect clinically significant thyroid cancers and guide optimal management of those nodules
- Dr. Wassner’s discussion focuses specifically on the different thyroid nodule cytologies according to the Bethesda classification system and the various recommended management strategies for each class.
- Historically, the evaluation of thyroid nodules occurred significantly less in children compared to adults. This was because the malignancy rates in children reached up to 70%, thus promoting the notion that all thyroid nodules in children are likely cancerous and should be resected without needing confirmatory biopsy.
- When biopsies began to be performed more consistently in children, the management process was variable between institutions until the official 2015 Management Guidelines for Children with Thyroid Nodules and Differentiated Thyroid Cancer was published in 2015. These guidelines stimulated the increased collection of pediatric data.
- With a consistent cytopathology workflow now established, Dr. Wassner assessed whether the malignancy rates in children and adults are similar within the Bethesda cytological categories.
- The results showed that the malignancy rates were insignificantly different between adults and children when considering the nodules that were originally deemed suspicious and malignant. However, in the indeterminate categories of AUS and follicular neoplasm, the pediatric malignancy rates were significantly higher.
- Performing repeat FNA biopsies of atypical cytologies in children altered the management plan 50% of the time. Thus, it is worthwhile to conduct a repeat FNA for children with indeterminate cytologies like AUS and follicular neoplasm.
- Dr. Bauer complimented Dr. Wassner’s findings with a discussion of when to perform FNA biopsies and how to improve the accuracy and reliability of results using molecular analysis.
- When comparing the ATA scoring guidelines for adult thyroid nodules versus the TI-RADS pediatric system, it seems that the extremes of the TI-RADS scoring system are accurate indicators of malignancy or lack thereof. However, the indeterminate nodules are still difficult to assess with both FNA and ultrasound. Additionally, nodule size should not be used to stratify pediatric patients.
- Dr. Bauer suggests that oncogene molecular testing is the answer to the question of which diagnostic method can best differentiate the indeterminate categories. The presence of an oncogenic driver increases the accuracy in diagnosis. The positive predictive value of malignancy was correlated to the level of oncogenic drivers, with the exception of RAS, which actually increased the benign call rate. This data needs to be further expanded upon in a prospective fashion.
Prevalence & Risk Factors for Multifocality in Pediatric Thyroid Cancer
Presenters: Drs. Grace Banik, Maisie Shindo & Gary Francis
Summary
Dr. Banik & Dr. Shindo present their excellent multicenter study to determine the critical predictors of multifocal disease in children with thyroid cancer.
- Dr. Banik begins with an overview of pediatric thyroid cancer. Pediatric PTC is a unique entity with distinct pathologic, molecular, and oncologic features compared to adults. Children tend to present with more advanced disease (3:31).
- Multifocal disease in one or more lobes tends to be more common in children than adults. This is an important factor to consider in management decisions pertaining to pediatric PTC (4:31).
- Recently, there has been a renewed interest in conservative approaches to managing pediatric PTC. Lobectomy can actually still be considered in children with small, solitary PTC (6:03).
- That being said, the notion of conservative surgery continues to be debated. Thus, Dr. Banik’s and Dr. Shindo’s attempts to further elucidate the prevalence and risk factors for multifocal disease in pediatric PTC (7:15).
- The significant predictors of multifocal disease were bilateral N1b, M1, and T3 stages, as well children under the age of 10 (11:44).
- The significant predictors of bilateral disease were NN1b and T3 stages, as well as children under the age of 10 (12:53).
- Ultimately, Dr. Banik’s study clarifies and strengthens the role of multifocality within the overall schema of surgical decision making for children with thyroid cancer.
- Dr. Francis offers his expert opinion on the study and elaborates further on the topic of pediatric thyroid cancer (20:45).
- Based on the findings from this study and prior studies, Dr. Francis concludes that bilateral disease is common in pediatric series and is most predicted by age, prior radiation, predisposing cancer syndromes, preoperative imaging, metastasis, N staging, extrathyroidal extension, and lymphovascular invasion (30:49).
- Additionally, Dr. Francis proposes a series of considerations that otherwise would warrant only a lobectomy in pediatric patients (31:31).
- More molecular research is needed to determine how the multifocal behavior of pediatric thyroid cancer differs from adults. This is particularly important to consider when discussing the possibility of over-aggressive surgical management of multifocal disease in children (44:03).

Add Upcoming TIROxMDS Seminars to Your Calendar
In 2 quick steps, you can add our entire schedule of upcoming seminars to your calendar today! Then you will get notified of upcoming lectures, presentations and case-based studies. Every Friday at 8:00 AM EST, we cover a new topic from published research.
Add to CalendarReferences
- 1.Haugen B, Alexander E, Bible K, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. doi:10.1089/thy.2015.0020
- 2.Ito Y, Onoda N, Okamoto T. The revised clinical practice guidelines on the management of thyroid tumors by the Japan Associations of Endocrine Surgeons: Core questions and recommendations for treatments of thyroid cancer. Endocr J. 2020;67(7):669-717. doi:10.1507/endocrj.EJ20-0025
- 3.Gharib H, Papini E, Garber JR, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules – 2016 Update Appendix. Endocrine Practice. Published online May 2016:1-60. doi:10.4158/ep161208.gl
- 4.Filetti S, Durante C, Hartl D, et al. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30(12):1856-1883. doi:10.1093/annonc/mdz400
- 5.Haddad RI, Nasr C, Bischoff L, et al. NCCN Guidelines Insights: Thyroid Carcinoma, Version 2.2018. J Natl Compr Canc Netw. Published online December 2018:1429-1440. doi:10.6004/jnccn.2018.0089
- 6.Tessler FN, Middleton WD, Grant EG, et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. Journal of the American College of Radiology. Published online May 2017:587-595. doi:10.1016/j.jacr.2017.01.046
- 7.Alexander E, Pearce E, Brent G, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid. 2017;27(3):315-389. doi:10.1089/thy.2016.0457