Initial Staging & Response to Therapy
Clinical Outline
= Primers in Thyroidology
= Journal Club Presentation
= Case-based Discussion (inc. roundtables)
= Grand Rounds Lectureship
1. Initial Staging & Assessing Response to Therapy
A. The Process of Staging a Patient
ATA // AAES // ESMO // JAES // NCCN
- Thyroid cancer management recommendations should be based on patient specific risk assessments.1–5
- AJCC/UICC TNM staging system is recommended for prediction of disease specific mortality.1–5
Tables & Figures
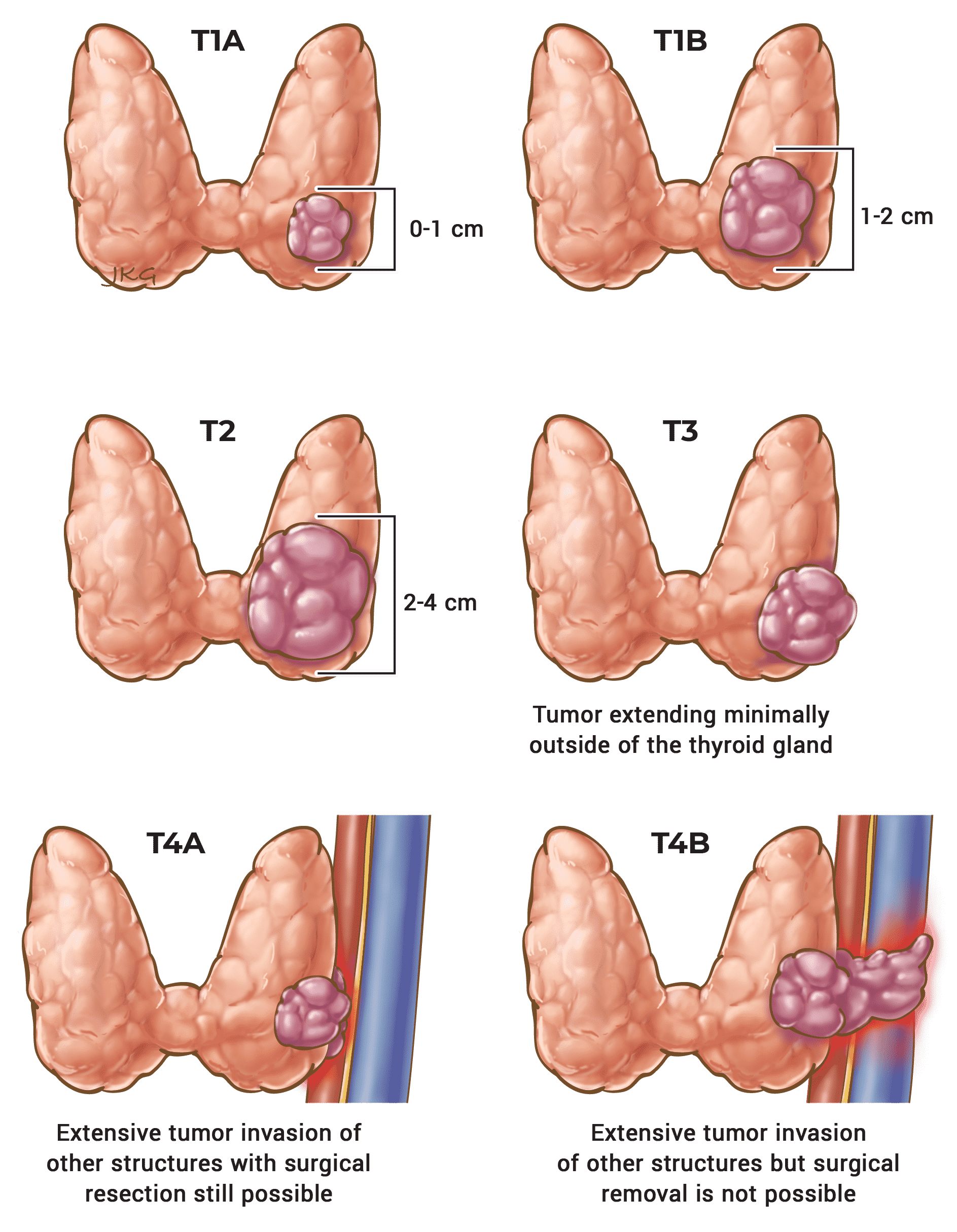
Table 5. AJCC Staging of Differentiated Thyroid Cancer (Disease Specific Mortality)
Age at Diagnosis
Younger than 55 Years
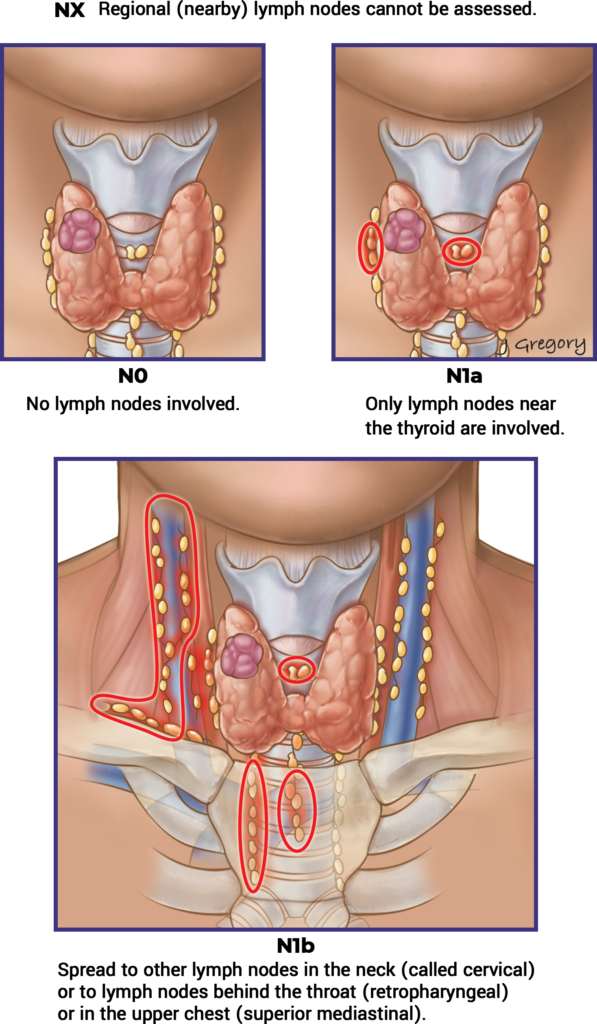
| T | N | M | Stage Group |
|---|---|---|---|
| Any T | Any N | M0 | I |
| Any T | Any N | M1 | II |
55 Years or Older
| T | N | M | Stage Group |
|---|---|---|---|
| T1 | N0 / NX | M0 | I |
| T1 | N1 | M0 | II |
| T2 | N0 / NX | M0 | I |
| T2 | N1 | M0 | II |
| T3a / T3b | Any N | M0 | II |
| T4a | Any N | M0 | III |
| T4b | Any N | M0 | IV A |
| Any T | Any N | M1 | IV B |
- A three-tiered risk stratification system (low, intermediate or high)1–3,5 or a four-tiered risk stratification system (very low, low, intermediate or high)4 is recommended for predicting the risk of having persistent or recurrent disease.
Tables & Figures
Table 11. Risk Stratification System with Proposed Modifications (ATA 2009)
Risk Level
ATA Low Risk
- Papillary thyroid cancer (with all of the following):
- No local or distant metastases,
- All macroscopic tumor has been resected,
- No tumor invasion of loco-regional tissues or structures,
- The tumor does not have aggressive histology (e.g. tall cell, hobnail variant, columnar cell carcinoma),
- If I131 is given, there are no RAI-avid metastatic foci outside the thyroid bed on the first post-treatment whole-body RAI scan,
- No vascular invasion,
- Clinical N0 or ≤ 5 pathologic N1 micrometastases (< 0.2 cm in largest dimension).*
- Intrathyroidal, encapsulated follicular thyroid variant of papillary thyroid cancer.*
- Intrathyroidal, well-differentiated follicular thyroid cancer with capsular invasion and no or minimal (< 4 foci) vascular invasion.*
- Intrathyroidal, papillary microcarcinoma, unifocal or multifocal, including BRAFV600E mutated (if known).*
ATA Intermediate Risk
- Microscopic invasion of tumor into the perithyroidal soft tissues.
- RAI-avid metastatic foci in the neck on the first post-treatment whole-body RAI scan.
- Aggressive histology (e.g. tall cell, hobnail variant, columnar cell carcinoma).
- Papillary thyroid cancer with vascular invasion.
- Clinical N1 or > 5 pathologic N1 with all involved lymph nodes < 3 cm in largest dimension.*
- Multifocal papillary microcarcinoma with ETE and BRAFV600E mutated (if known).*
ATA High Risk
- Macroscopic invasion of tumor into the perithyroidal soft tissues (gross ETE).
- Incomplete tumor resection.
- Distant metastases.
- Postoperative serum thyroglobulin suggestive of distant metastases.
- Pathologic N1 with any metastatic lymph node ≥ 3 cm in largest dimension.*
- Follicular thyroid cancer with extensive vascular invasion (> 4 foci of vascular invasion).*
Figure 11. ATA Spectrum of Risk of Recurrence
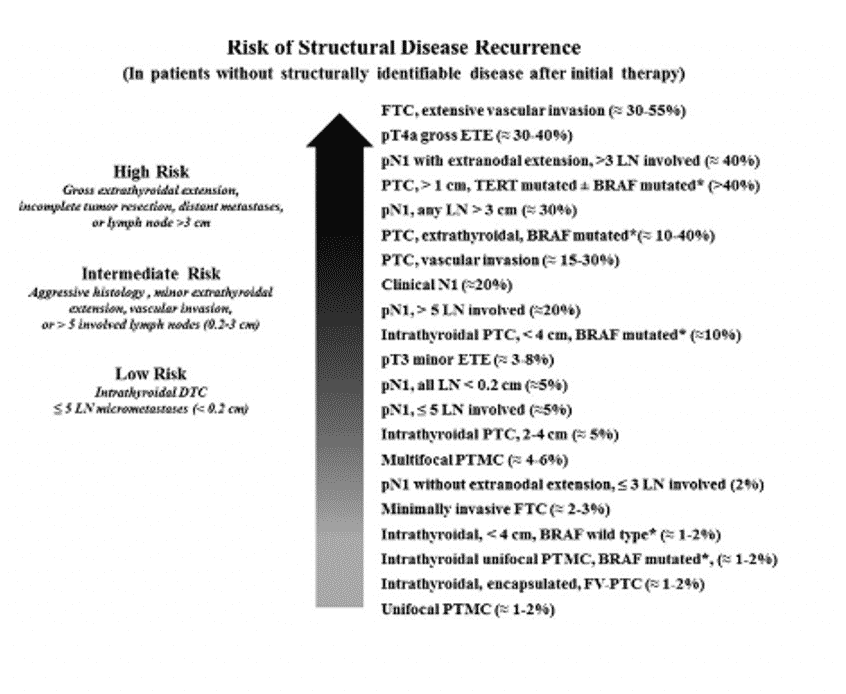
- Recognizing that the risk of structural disease recurrences is best thought of as a continuum rather than as discrete risk categories, the use of additional prognostic variables is recommended to further refine individual risk estimates.1–5
Factors Associated with Increasing Risk of Disease Recurrence1–5- Age at diagnosis. Recurrence rates higher at extremes of age.
- Increasing size of the primary tumor.
- Increasing size of cervical lymph node metastases.
- Increasing number of cervical lymph node metastases.
- Extranodal extension in lymph node metastases.
- Increasing extent of vascular invasion. Greater than 4 foci.
- Potentially more aggressive histology. For example, tall cell variant papillary thyroid cancer, diffuse sclerosing variant papillary thyroid cancer, Hürthle cell carcinoma and poorly differentiated thyroid cancer.
- Potentially aggressive mutational profile. For example, BRAFV600E + TERT promoter mutation, or RAS + TERT promoter mutation.
- Abnormal postoperative thyroglobulin at least 4–6 weeks after thyroid surgery.
- Abnormal findings on post-operative diagnostic or post-radioactive iodine (I131) therapy whole body scans (if done).
Supplemental Educational Content
Optimal Age Cutoff for the UICC/AJCC TNM Staging System
Presenter: Dr. Evert van Velsen
Summary
Dr. Evert van Velsen presents an article entitled, “Finding the optimal age cutoff for the UICC/AJCC TNM staging system in patients with papillary or follicular thyroid cancer.” Dr. Erik Verburg joins as the Featured Guest Discussant.
- Dr. Evert van Velsen compares the eighth edition of the UICC/AJCC TNM (tumor, node, metastasis) to the seventh edition. (2:55) One of the major differences between the 7th and 8th edition is that the age cutoff was changed from 45 to 55 years of age. This was based on the findings in three previous studies that showed a 55 year age cutoff led to better prediction of disease specific survival (DSS) in patients with differentiated thyroid cancer. However, these studies were based on the 7th edition UICC/AJCC TNM staging, not the updated 8th edition.
The aim of this study was two-fold:
- Investigate the optimal age cutoff for the TNM staging system to predict DSS using the 8th edition UICC/AJCC TNM staging system.
- Evaluate whether the optimal age cutoffs for follicular thyroid cancer (FTC) and papillary thyroid cancer (PTC) differ.
- This study concluded that when using five-year increments, 50 years is the optimal age cutoff for differentiated thyroid cancer (DTC) and PTC, but 40 years is the optimal age cutoff for FTC. When using one-year increments, 50 years is the optimal age cutoff for DTC, 48 years is the optimal age cut-off for PTC, and 41 years is the optimal age cut-off for FTC.
Additional conclusions provided by this study:
- Age-based cutoffs improve the prognostic power of the TNM staging system. However, whether or not a dichotomous age cutoff is the optimal way to incorporate age into these classification systems is unknown.
- PTC and FTC should be staged separately.
- Dr. Erik Verburg discusses the important lessons that can be learned from big databases. (21:50)
- Dr. Verburg summarizes the purpose of staging systems. Staging systems categorize individual patients into discrete risk categories, which allow us to determine which line of treatment best suits the patient. (23:20)
- Dr. Verburg describes the challenges associated with comparing different staging systems, highlighting the array of methodologies used and lack of consensus on how to do so. (27:14)
- Looking at long-term follow-up of patients is important when comparing staging models and identifying subtle effects.
- Dr. van Velsen provides possible explanations for how age might play a role in the inherent biological differences between papillary and follicular thyroid carcinoma. (45:21)
Defining Advanced Thyroid Cancer & Its Targeted Treatment
Presenter: Dr. David C. Shonka, Jr.
Summary
- 3:40 – Targeting Driver Mutations for Treatment
Dr. Shonka provides some background on how driver mutations are associated with specific subtypes of thyroid cancer, and how they can be targeted for treatment. - 9:09 – Putting Together a Statement
Dr. Shonka discusses how the consensus statement was put together by a multidisciplinary team of panelists who voted on various statements related to the treatment of advanced thyroid cancer. - 13:28 – The Mutational Testing Process
Dr. Shonka presents figures from the paper summarizing how panelists believe mutational testing should be approached for advanced differentiated thyroid cancer. - 16:14 – Mutational Testing for Thyroid Cancer Subtypes
Dr. Shonka summarizes recommendations for mutational testing when treating medullary thyroid carcinoma and anaplastic thyroid carcinoma. - 19:05 – Clinical Implications
Dr. Shonka concludes with an overview of targeted systemic therapy options, practical impacts, and study conclusions. - 21:17 – Case Study
Dr. Shonka presents a case illustration to demonstrate how mutational testing can change the way providers manage advanced thyroid cancer treatment.
Variation in the Diagnosis of Noninvasive Follicular Thyroid Neoplasm With Papillary-like Nuclear Features (NIFTP)
Presenter: Debbie Chen, MD
Summary
- 9:25 – Revised Diagnostic Criteria for NIFTP
Dr. Chen discusses a recent study by Nikiforov et al. that proposed stricter diagnostic criteria for noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP). This study led to the North American Association of Central Cancer Registries (NAACCR) modifying its coding scheme. Noninvasive EFVPTC was reclassified as NIFTP. - 13:27 – Trends in TC Incidence Rate
Dr. Chen presents trends in the incidence rates of thyroid cancer by subtypes and NIFTP. Of many notable trends, we can see that there was a statistically significant increase in the incidence of general PTC. - 24:21 – Multifocal Tumors
Dr. Baloch details how the presence of a second tumor in the thyroid gland does not change the diagnosis of NIFTP.
Work in Progress – A Computational Approach to Evaluating Tallness in Papillary Thyroid Carcinoma
PresenterS: Margaret Brandwein-Weber, MD & Scott Doyle, PhD
Summary
- 7:41 — Tall Cell Variant (TCV) as a Poor Prognosticator
Dr. Brandwein discusses previous studies that used multivariate analysis to investigate outcomes associated with TCV. - 13:04 — Study Presentation
Dr. Brandwein presents her study investigating TCV in primary papillary thyroid carcinoma (PTC), highlighting methodology and results. - 20:44 — Assessing Tallness with Computerized Systems
Dr. Brandwein explains how mathematical functions and computer software can be used to assess tallness more accurately compared to the traditional “eyeball” method. - 32:57 — Quantifying Tallness
Dr. Doyle explains the principles and conceptual framework behind training artificial intelligence (AI) systems to quantify tallness. - 38:20 — Training the AI Classifier
Dr. Doyle discusses how pathological annotation of patient slides were used to create “tiles” for training the AI classifier.
Challenges in Thyroid Cancer Surgery: Case Based Presentation to a Panel of International Leaders
Moderator: Dr. Mark L. Urken
Panelists: Drs. Gregory Randolph, Joseph Scharpf, Ashok Shaha and Mark Zafereo
Summary
- 12:33 — Mgmt of Functioning RLN When Cancer “Involved”
Dr. Urken presents the decision-making process when observing adherence of a nerve on a lymph node. Dr. Shaha explains to what extent he will go to preserve the function of this nerve and explains that he would not sacrifice the nerve. Dr. Randolph explains that nerve resection is not a consideration for this patient in terms of recurrence or overall survival. This nerve must be judicially dissected to ensure that it is invaded. - 24:05 — Value of Frozen Section in Surgery
Dr. Urken presents a case of right thyroid mass and paralyzed right vocal cord. Preoperative imaging suggested a high grade lesion. In this case, Dr. Scharpf discusses how he would use frozen section to guide the extent of his surgery. Prognostic and therapeutic information can also be gained from frozen section analysis. - 30:21 — Mgmt of Recurrent Disease
Dr. Urken begins discussion on the topic of removing recurrent nodal disease for a 33-year-old female who underwent total thyroidectomy at an outside institution with positive nodes. For Dr. Scharpf, the prior finding of ENE does not necessarily impact decisions. For Dr. Zafereo, surgery and surveillance are the only two options for managing this patient. Dr. Zafereo also suggests closer inspection of imaging. - 42:25 — Neoadjuvant Therapy in BRAFv600e Disease
In this case, Dr. Zafereo describes how he would proceed with better understanding the patient’s histopathology. He suggests a BRAF inhibitor for this patient. Dr. Randolph also suggests trying a neoadjuvant approach.
Supplemental Educational Content
Table 7. Gene Alterations & Histology
Phenotype
Toxic Adenoma
Known Somatic Genetic Mutation / Alterations
- TSH-R
- GNAS
Benign Thyroid Nodules
Known Somatic Genetic Mutation / Alterations
- N-, H- and K-RAS
- EIF1AX
Noninvasive Follicular Tumor with Papillary-like Features (NIFTP)
Known Somatic Genetic Mutation / Alterations
- N-, H- and K-RAS
- BRAF K601E
Infiltrative Follicular Variant Papillary Thyroid Carcinoma (FVPTC)
Known Somatic Genetic Mutation / Alterations
- N-, H- and K-RAS
- BRAF V600E
Papillary Thyroid Carcinoma (PTC)
Known Somatic Genetic Mutation / Alterations
- RET/PTC
- BRAF V600E
- N-, H-, K-RAS
- TERT
Columnar Cell, Tall Cell, Hobnail Variant PTC
Known Somatic Genetic Mutation / Alterations
- BRAF V600E
Diffuse-sclerosing Variant PTC
Known Somatic Genetic Mutation / Alterations
- RET/PTC
Follicular Thyroid Carcinoma (FTC)
Known Somatic Genetic Mutation / Alterations
- N-, H- and K-RAS
- PAX8/PPAR
- PTEN
Hürthle Cell Carcinoma
Known Somatic Genetic Mutation / Alterations
- NRAS
- Genes in the PI3K-Akt Pathway
Poorly Differentiated Thyroid Carcinoma (PDTC)
Known Somatic Genetic Mutation / Alterations
- N-RAS (Insular)
- BRAF V600E
- PIK3CA
- RET/PTC
- TERT
Anaplastic Thyroid Carcinoma (ATC)
Known Somatic Genetic Mutation / Alterations
- N-RAS, BRAF V600E
- PIK3CA
- TP53
- β-catenin
- EIF1AX
Medullary Thyroid Carcinoma (MTC)
Known Somatic Genetic Mutation / Alterations
- RET (Germ Line Mutation in Inherited MTC, Somatic Mutation)
- N-, H- and K-RAS (Somatic Mutations)
Adapted with permission from Wolters Kluwer Health, Inc: the author(s), titles of article, title of journal, volume number, issue number, inclusive pages and website URL to the journal page.
Table 8. Histologic Subtypes of PTC
Subtype
Follicular-variant
| Nuclear Features of PTC Present? |
| Yes |
| Characteristics |
| • RAS mutation more common. • Follicular growth pattern. |
| Prognosis |
| 10-year DSS 93% |
Follicular-variant Encapsulated, Invasive
| Nuclear Features of PTC Present? |
| — |
| Characteristics |
| • RAS mutation or PPARG rearrangement more common. • Low rate of LNM. |
| Prognosis |
| 10-year DSS ~100% |
Follicular-variant Nonencapsulated / Infiltrative
| Nuclear Features of PTC Present? |
| — |
| Characteristics |
| BRAF V600E more common. |
| Prognosis |
| Equivalent to classic / conventional PTC |
Columnar Cell
| Nuclear Features of PTC Present? |
| No |
| Characteristics |
| • Papillae lined by columnar cells with nuclear stratification. • Large tumors with capsular invasion are associated with LNM and DM. |
| Prognosis |
| Variable |
Cribiform Morular
| Nuclear Features of PTC Present? |
| Occasionally |
| Characteristics |
| • Presence of morules—squamoid areas with intranuclear inclusions and nuclear clearing. • Associated with Familial Adenomatosis Polyposis Syndrome. |
| Prognosis |
| Equivalent to classic / conventional PTC |
Classic / Conventional
| Nuclear Features of PTC Present? |
| Yes |
| Characteristics |
| LNM Common |
| Prognosis |
| • 5-year DSS 97.4% • 10-year DSS 93% |
Diffuse Sclerosing
| Nuclear Features of PTC Present? |
| Yes |
| Characteristics |
| • Diffuse fibrosis. • Dense lymphoid infiltration. • Squamous metaplasia. |
| Prognosis |
| • 5-year DSS 96% • Equivalent to high-risk PTC |
Tall Cell
| Nuclear Features of PTC Present? |
| Yes |
| Characteristics |
| • Extrathyroidal extension and LNM possible. • > 30% cells are 2x tall as wide and eosinophilic cytoplasm. |
| Prognosis |
| 5-year DSS 95.6% |
Hobnail
| Nuclear Features of PTC Present? |
| Yes |
| Characteristics |
| • Increased risk of DM. • > 30% have hobnail features (eccentric nuclei and tapering cytoplasm). • Syncytial or micropapillary clusters with apically placed nuclei. • BRAF V600E or p53 positive |
| Prognosis |
| 5-year DSS 83% |
Adapted with permission from Wolters Kluwer Health, Inc.: the author(s), titles of article, title of journal, volume number, issue number, inclusive pages and website URL to the journal page.
- The lowest risk of recurrence is associated with classic papillary thyroid cancer:
- ≤ 1 cm.
- Confined to the thyroid.
- Without clinically apparent lymph node metastasis.
- Initial risk estimates should be continually modified during follow-up as additional data is accumulated.1,3,5
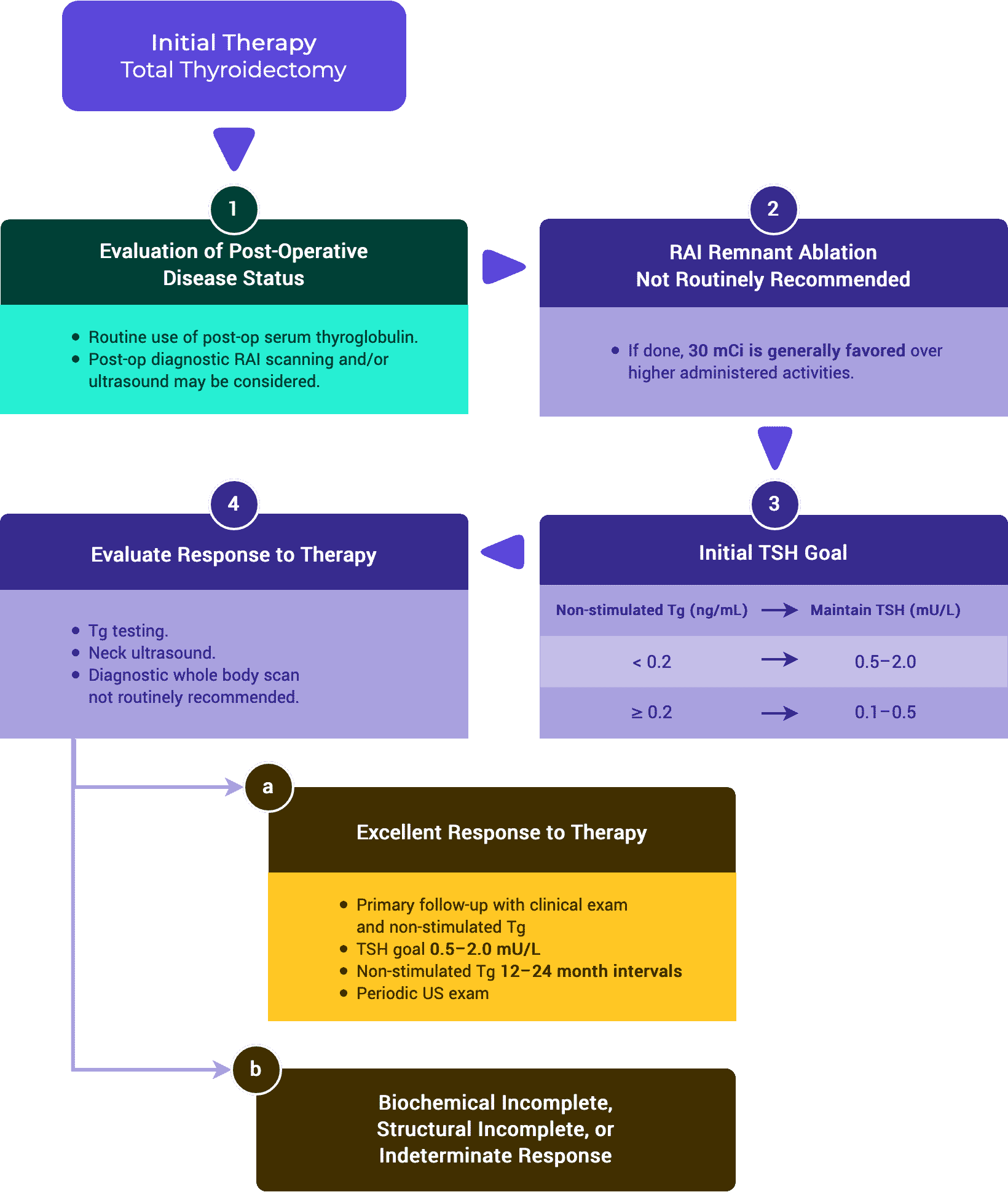
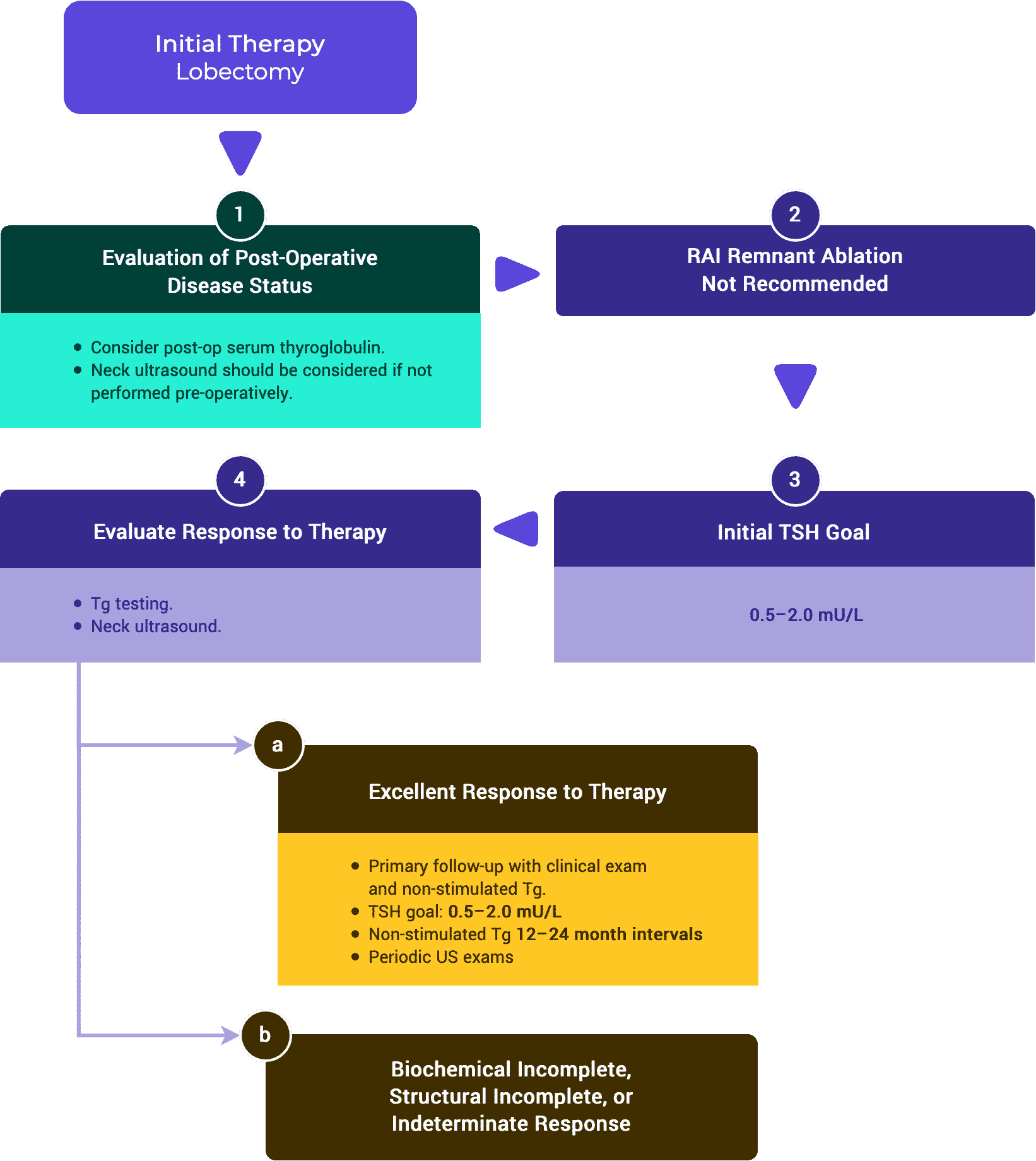
- One method to facilitate ongoing risk assessment is to classify patients at each follow up based on their response to therapy as having either an:1,5
- Excellent Response. No clinical, biochemical, or structural evidence of disease.
- Biochemical Incomplete Response. Abnormal Tg or rising anti-Tg antibodies in the absence of localizable disease.
- Structural Incomplete Response. Persistent or newly identified loco-regional or distant metastases.
- Indeterminate Response. Non specific biochemical or structural findings that cannot be confidently classified as either benign or malignant including patients with stable or declining anti-Tg antibodies without definitive structural evidence of disease.
Tables & Figures
Table 2a. Response to Treatment: Total Thyroidectomy & RRA (ATA vs. ESMO)
Response to Treatment
Excellent
| Imaging |
| No relevant findings on imaging. |
| TSH-Suppressed Tg |
| < 0.2 ng/mL |
| TSH-Stim TG |
| < 1 ng/mL |
| TgAB |
| • Not specified (ATA) • Undetectable (ESMO) |
Biochem Incomplete
| Imaging |
| No relevant findings on imaging. |
| TSH-Suppressed Tg |
| 1 ng/mL |
| TSH-Stim TG |
| 10 ng/mL |
| TgAB |
| Rising TgAB levels |
Structural Incomplete
| Imaging |
| Evidence of structural or functional disease. * |
| TSH-Suppressed Tg |
| * |
| TSH-Stim TG |
| * |
| TgAB |
| * |
Indeterminate
| Imaging |
| Nonspecific findings on US/CT. — OR — Faint uptake in thyroid bed on RAI scanning. |
| TSH-Suppressed Tg |
| • Tg detectable, but < 1 ng/mL (ATA) • 0.2–1 ng/mL (ESMO) |
| TSH-Stim TG |
| • Stimulated Tg detectable, but < 10 ng/mL (ATA) • 1–10 ng/mL (ESMO) |
| TgAB |
| TgAB levels stable or declining in the absence of structural or functional disease. |
* Regardless of Tg or TgAB levels.
Table 2b. Response to Treatment: Total Thyroidectomy Alone
Response to Treatment
Excellent
| Imaging |
| No relevant findings on imaging. |
| TSH-Suppressed Tg |
| < 0.2 ng/mL |
| TSH-Stim TG |
| Not Specified |
| TgAB |
| Undetectable |
Biochem Incomplete
| Imaging |
| No relevant findings on imaging. |
| TSH-Suppressed Tg |
| Tg > 5 ng/mL – OR – Rising Tg values with similar TSH levels. |
| TSH-Stim TG |
| — |
| TgAB |
| Rising TgAB levels |
Structural Incomplete
| Imaging |
| Evidence of structural or functional disease. * |
| TSH-Suppressed Tg |
| * |
| TSH-Stim TG |
| * |
| TgAB |
| * |
Indeterminate
| Imaging |
| Nonspecific findings on US/CT. — OR — Faint uptake in thyroid bed on RAI scanning. |
| TSH-Suppressed Tg |
| < 0.2–5 ng/mL |
| TSH-Stim TG |
| Not Specified |
| TgAB |
| TgAB levels stable or declining in the absence of structural or functional disease. |
* Regardless of Tg or TgAB levels.
Table 2c. Response to Treatment: Lobectomy Alone
Response to Treatment
Excellent
| Imaging |
| No relevant findings on imaging. |
| TSH-Suppressed Tg |
| Stable |
| TSH-Stim TG |
| Stable |
| TgAB |
| Undetectable |
Biochem Incomplete
| Imaging |
| No relevant findings on imaging. |
| TSH-Suppressed Tg |
| Rising Tg values with similar TSH levels |
| TSH-Stim TG |
| TgAB |
| Rising |
Structural Incomplete
| Imaging |
| Evidence of structural or functional disease. * |
| TSH-Suppressed Tg |
| * |
| TSH-Stim TG |
| * |
| TgAB |
| * |
Indeterminate
| Imaging |
| Nonspecific findings on US/CT. |
| TSH-Suppressed Tg |
| * |
| TSH-Stim TG |
| * |
| TgAB |
| * |
* Regardless of Tg or TgAB levels.
Supplemental Educational Content
Interobserver Variability in the Histopathologic Assessment of Extrathyroidal Extension of Well Differentiated Thyroid Carcinoma
Presenters: Juan Hernandez-Parra, MD & Dominick Guerrero, MD
Commentary: Margaret Brandwein-Weber, MD
Study
- 11 expert pathologists reviewed 69 scanned slides chosen for potential controversy with minimal extrathyroidal extension (mETE).
- Found significant interobserver variability in histopathologic assessment of ETE.
Conclusion
- Microscopic identification of ETE is subjective, particularly from consideration of only adipose tissue, vasculature, or nerves rather than skeletal muscle.
- Complexity in part due to unique histologic, anatomic, and developmental properties of the thyroid.
Applied to AJCC 8th Edition
According to the authors, the results support the relatively strict criteria for ETE included in the 8th edition of the AJCC Cancer Staging Manual.
- “May decrease interobserver variability”
- Allows greater consistency in diagnosis and staging of thyroid carcinoma.
While interobserver variability is solved by 8th AJCC, this calls into question many studies examining the impact of ETE.
Impact of AJCC 8th Edition
- Solved interobserver variability, to some extent.
- Increased the need for enhanced communication between surgeons and pathologists.
- Created a disconnect with the ATA guidelines.
Remaining Controversy
- Neither AJCC nor CAP offer guidance if multifocal extensive mETE are not recognized grossly.
- mETE has an association with aggressive biology; associated with ENE, positive lymph nodes and ATA aggressive lymph node classification.
Real-World Performance of the American Thyroid Association Risk Estimates in Predicting 1-Year Differentiated Thyroid Cancer Outcomes
Presenters: Cosimo Durante, MD, PhD
A Prospective Multicenter Study of 2000 Patients
Dr. Cosimo Durante and Dr. Amanda La Greca discuss the American Thyroid Association risk classification system as a tool to differentiate between risks of recurrent disease and to decrease overdiagnosis and overtreatment for thyroid cancer patients.
- Before Dr. Durante’s study, the efficacy of the 2015 ATA risk stratification system had not yet been tested in real-world settings (15:21)
- This study proved that the ATA risk classification system works and is a significant predictor in response to treatment at the 1-year follow-up for patients with differentiated thyroid cancer
- Dr. Amanda La Greca discusses the “broken chair” concept that occurs when inadequate patient information is provided at the initial visit (40:03)
- Dr. La Greca emphasizes the importance of distinguishing and diagnosing aggressive thyroid cancers that necessitate intensive therapeutic efforts and close post-treatment surveillance (46:42)
A Retrospective Cohort Study with Validation of Predictors of Differentiated Thyroid Cancer Outcomes
Presenters: Ayanthi Wijewardene, MD
- 4:30: Stratifying Very Low Risk Patients
Dr. Wijewardene discusses why current ATA risk stratification guidelines don’t adequately identify very low risk patients who may not require radioactive iodine (RAI) treatment. - 8:00: Revising a Risk Model
Dr. Wijewardene explains how she and her team identified variables associated with metastasis or recurrence and developed a predictive risk model incorporating those factors. - 12:47: Building Upon the ATA Guidelines
Dr. WIjewardene summarizes the results and conclusions of her study, emphasizing how her proposed Predictive Risk Model identified more patients with synchronous metastases or recurrence compared to ATA risk stratification system.
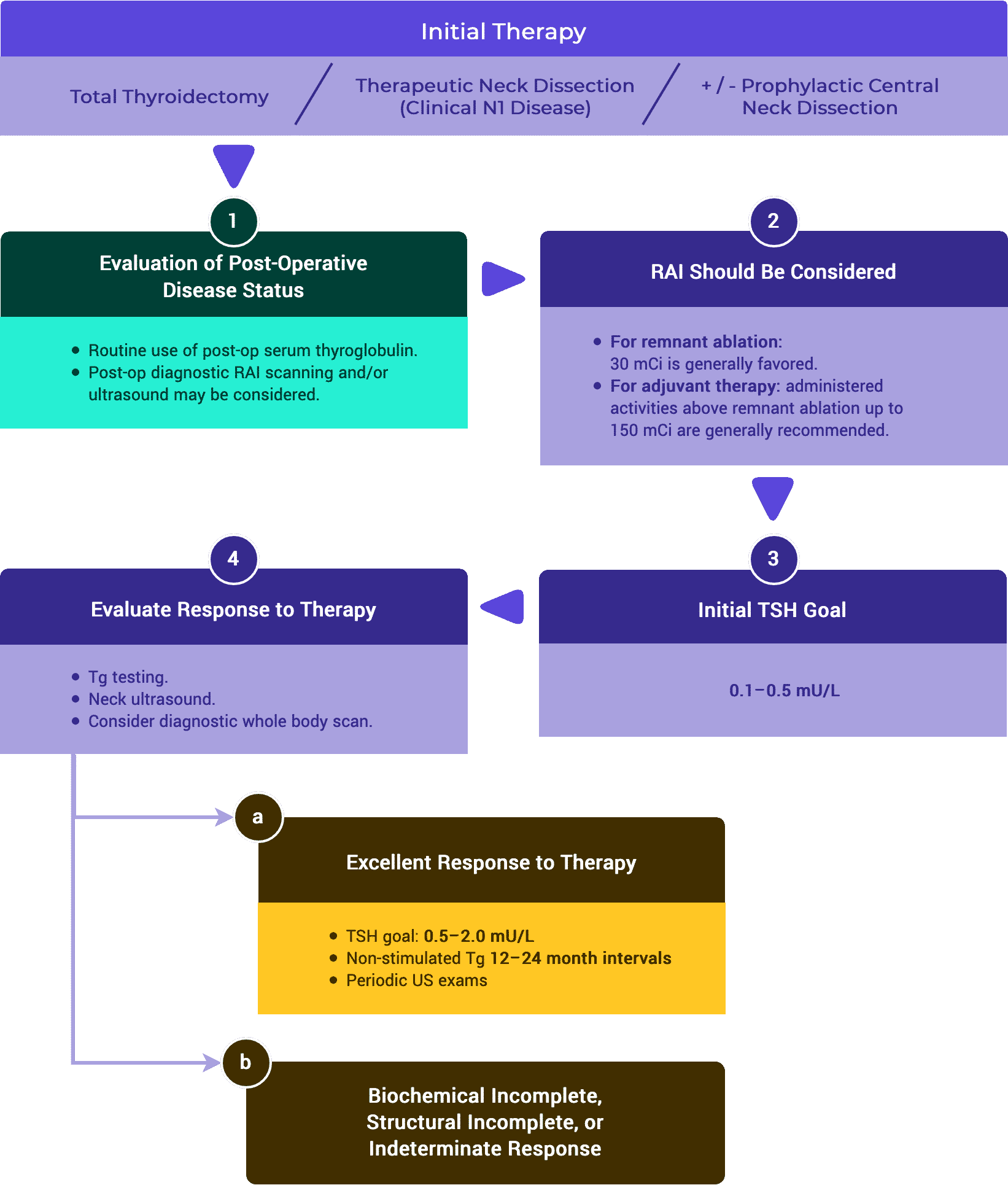
2. Follow-up Recommendations After Response to Therapy is Established
A. Excellent Response to Therapy
ATA // ESMO // NCCN
In low and intermediate risk patients, an excellent response to therapy should lead to an early decrease in the intensity and frequency of follow-up and the degree of TSH suppression.1,3,5
Supplemental Educational Content
Dynamic Risk Assessment in Patients with Differentiated Thyroid Cancer
Presenter: Fabian Pitoia, MD
Dr. Fabian Pitoia and Dr. Iwao Sugitani discuss the critical role of dynamic risk assessment in decision-making for differentiated thyroid cancer care.
Summary
Dr. Fabian Pitoia presents the concept of the “broken chair” in regard to patients with differentiated thyroid cancer, highlighting that one does not always have full information about the initial risk of recurrence when one of the “legs” is missing. These “legs” include:
- Information about the surgical procedure
- Detailed pathological report
- Reliable image studies
- Reliable lab tests
Dr. Fabian Pitoia explains that one moves from a “broken chair” to a “repaired chair” when there is access to responses to treatment. This is the change from static risk assessment to dynamic risk assessment.
- This helps to predict a reduced probability of SIR.
Dr. Iwao Sugitani emphasizes the importance of conducting risk assessment throughout the whole process of diagnosis, surgery, adjuvant therapies, and follow-up. This risk assessment includes:
- Identification of low risk patients that might qualify for active surveillance and high risk patients that require a classic treatment regimen in the peri-diagnostic period.
- Mortality-risk assessment and recurrence risk assessment at the time of initial decision-making in treatment of PTC, including surgery and adjuvant therapies.
- Response to therapy assessment during follow-up
i. Follow-up Intervals
- In ATA low and intermediate risk patients, the time interval between Tg measurements can be lengthened to at least 12–24 months.1,3,5
- Regardless of response to initial therapy, ATA high risk patients should have Tg determinations done at 6–12 month intervals for several years.1,5
TSH stimulated Tg testing is not recommended for low and intermediate risk patients with an excellent response to therapy.1,3,5
Non-stimulated Tg measurements should be considered in patients treated with either lobectomy or total thyroidectomy without radioactive iodine therapy.1,5
Serum TSH should be measured at least every 12 months in all patients on thyroid hormone therapy.1,3
ii. TSH Goals
- In low and intermediate risk patients demonstrating an excellent response to therapy TSH goal can be in the low reference range (0.5–2 mU/L).1,3,5
- In high risk patients with an excellent response to therapy, the ATA guidelines recommend keeping the TSH between 0.1–0.5 mU/L for up to 5 years, while the ESMO guidelines recommends a TSH goal of 0.5–2 mU/L.1,5
Neck ultrasound can be done periodically depending on the patient’s risk for recurrence and Tg status.1,3,5
- Following total thyroidectomy and radioactive iodine remnant ablation, low risk patients that achieve an excellent response can be followed primarily with clinical examination.1
- In high risk patients that achieve an excellent response to therapy, periodic surveillance using neck ultrasound, CT scans, and/or MRI scans can be considered.1,5
Supplemental Educational Content
Thyrotropin Suppression for Papillary Thyroid Cancer
Presenter: Maria Papaleontiou, MD
A Physician Survey Study
Drs. Maria Papaleontiou and Angela Leung discuss the spectrum of physician recommendations for use of thyroid stimulating hormone (TSH) suppression in patients with papillary thyroid cancer (PTC) in the context of one study and the literature at large.
Dr. Maria Papaleontiou presents findings from a study which sought to evaluate physicians’ recommendations with regard to use of TSH suppression after initial treatment in patients with PTC of varying degrees of risk:
- She noted that those physicians who see higher numbers of thyroid cancer patients were less likely to recommend TSH suppression in low-risk and very-low risk scenarios.
Dr. Maria Papaleontiou highlights that, given the risks and minimal benefits that TSH suppression presents to low-risk and very-low risk PTC patients, there is a need for educational initiatives directed toward low-volume physicians to reduce overtreatment in this realm.
Dr. Angela Leung provides an overview of outcomes related to use of TSH suppression in the literature, drawing attention to the inconsistent results and further need for prospective studies.
While discussing use of TSH suppression in elderly patients, Dr. Angela Leung notes that serum TSH in individuals of older age naturally increases over time.
iii. Diagnostic Whole Body Scan
- In low risk or intermediate risk patients achieving an excellent response to therapy, routine diagnostic whole body radioactive iodine scans are not recommended.1,3,5
- In ATA high risk or in intermediate risk with higher risk features, a diagnostic whole body radioactive iodine scan may be useful.1,3
B. Indeterminate Response to Therapy
ATA // ESMO // NCCN
Serum Tg on thyroxine therapy should be measured every 6–12 months. More frequent Tg measurements may be appropriate for ATA high-risk patients.1,3,5
i. TSH Goals
- It is generally recommended to maintain thyroid hormone therapy at a level to achieve serum TSH levels in a range of 0.1–0.5 mU/L for up to 5 years.1,5
- In patients initially classified as low risk for recurrence, the serum TSH may be targeted within the low reference range (0.5–2 mU/L).1
- After five years, the degree of TSH suppression can be reconsidered and potentially reduced with close surveillance.1
Cervical US should be performed every 6–12 months.1,5
In patients with abnormal or indeterminate US findings, TSH-stimulated radioiodine whole body scans together with Tg and Tg antibodies measurement can be considered.3
In patients with elevated serum Tg or rising Tg antibodies with or without negative RAI imaging, CT scan of the chest and/or 18 FDG-PET should be considered.1,3,5
Recurrence risk estimates should be reassessed and modified during follow-up.1,5
Nonspecific findings that become suspicious over time or rising Tg or anti-Tg antibody levels can be further evaluated with additional imaging or biopsy when feasible.1
If a positive result would change management, ultrasonographically suspicious lymph nodes ≥ 8–10 mm in the smallest diameter should be biopsied for cytology with Tg measurement in the needle washout fluid.1
Suspicious lymph nodes < 8–10 mm in the smallest diameter may be followed without biopsy with consideration for FNA or intervention if there is growth or if the node threatens vital structures.1
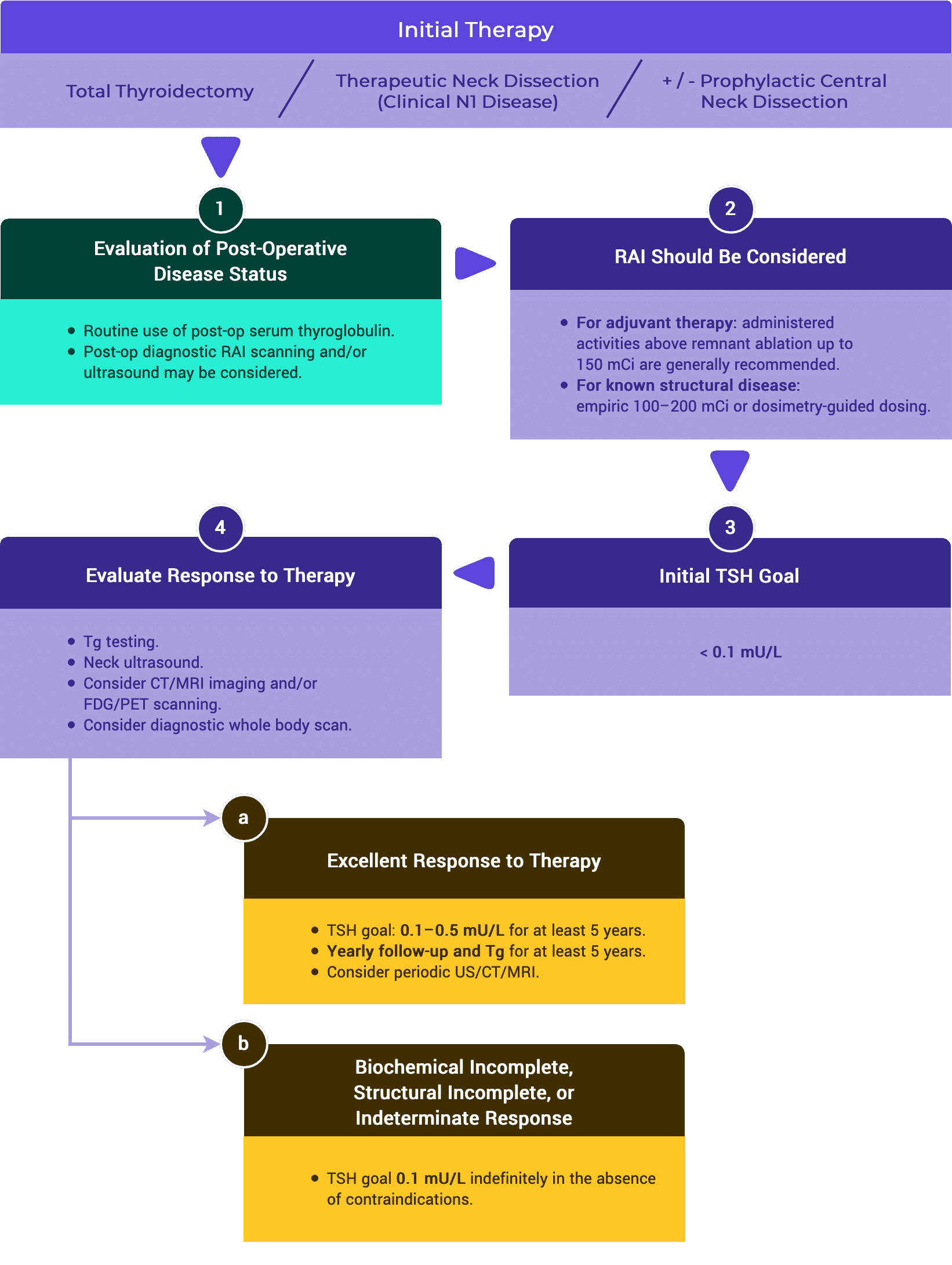
C. Biochemical Incomplete Response to Therapy
ATA // ESMO // NCCN
Follow-up should be performed at least every 6–12 months for several years, and then periodically depending on the patient’s risk and Tg status.1,3,5
Serum TSH should be maintained between 0.1 and 0.5 mU/L, taking into account the initial ATA risk classification, Tg level, Tg trend over time, and risks associated with TSH suppression.1,5
Neck ultrasonography is the main surveillance tool to identify either tumor bed recurrence or lymph node disease.1,3,5
Stimulated Tg testing (following either recombinant human TSH or thyroid hormone withdrawal) may be considered in patients with a biochemical incomplete response following either additional therapies or a spontaneous decline in Tg over time in order to reassess response to therapy.1
Functional assessment with TSH stimulated RAI imaging with either I123 (preferable as it avoids stunning) or low activity (2–5 mCi or 74–185 MBq) I131 with SPECT/CT may be performed to determine suitability for RAI therapy.1,3,5
F-18 FDG PET/CT ( with or without TSH stimulation) may be performed particularly for patients with negative RAI imaging but also in patients suspected of de-differentiated disease who may have neoplastic foci with no RAI uptake. FDG PET positive lesions are associated with a worse prognosis and RAI refractory disease.1,3,5
If further structural assessment is to be undertaken, then CT with iodine contrast or MRI is available. Chest CT is undertaken for the diagnosis of pulmonary and/or mediastinal metastases. MRI brain, MRI skeletal survey and CT or MRI abdomen may be required if patients have symptoms referable to these organs or prior to RAI therapy and are at risk of complications of tumor swelling. If iodinated contrast is administered, then there is a need to defer RAI assessment for 6–8 weeks.1,5
Rising Tg or TgAb indicates need for imaging and consideration of additional therapy. 1,2,3
D. Structural Incomplete Response to Therapy
ATA // ESMO // JAES // NCCN
Serial monitoring is generally recommended every 6 months or less.1,3–5
Patients with structurally recurrent or persistent disease should receive suppressive TSH therapy with a goal < 0.1 mIU/L.1,3
Identification of structural recurrent or persistent disease (incomplete response) is confirmed most commonly through abnormal imaging findings with subsequent USg-FNA. There may be times when USg-FNA is not possible or not needed.1–4
If a positive result would change management, ultrasonographically suspicious lymph nodes ≥ 8–10 mm in the smallest diameter should be biopsied for cytology with Tg measurement in the needle washout fluid.1
Suspicious lymph nodes < 8–10 mm in the smallest diameter may be followed without biopsy with consideration for FNA or intervention if there is growth or if the node threatens vital structures.1
Structural persistent or recurrent disease that is followed conservatively should be monitored through regular periodic assessment using:
- clinical evaluation,
- imaging (primarily ultrasound), and
- thyroglobulin and thyroglobulin antibodies measurement.1,3–5
Tables & Figures
Supplemental Educational Content
The Role of Thyroglobulin & Thyroglobulin Antibodies
Presenter: Eyal Robenshtok, MD
Dr. Robenshtok and Dr. Tuttle discuss the reliability of thyroglobulin and thyroglobulin antibodies in predicting long-term outcomes measures following thyroid lobectomy surgery.
Summary
- Dr. Robenshtok discusses his research study that focused on the standard thyroglobulin threshold—30 nanograms/milliliter—to determine the normal range and prognostic value of thyroglobulin following a lobectomy.
- The cohort demonstrated promising thyroglobulin levels one year after surgery. The average thyroglobulin was 12.1 ng/ml, well below the upper threshold for healthy activity.
- There was also no correlation between thyroglobulin levels and patient sex/age.
- To assess the prognostic value of thyroglobulin, the rate of recurrences were analyzed. While the recurrent group had a higher average levels of thyroglobulin after a year when compared to the non-recurrent group, the standard deviations were both lead to significant overlap. Thus, it is impossible to use the thyroglobulin levels to discern which patient would have recurrence after a year.
- Additionally, the dynamics of thyroglobulin antibodies were also not predictive of recurrence rate. This holds true for both subgroups of patients—Hashimoto’s thyroiditis and contralateral lobectomie.s
- Dr. Tuttle verified these findings with his own experience and research, stating that using thyroglobulin levels as an individual stand-alone marker has poor specificity and poor sensitivity in the setting of a lobectomy. That being said, it should not be totally discarded and should be considered when the numbers are well above the threshold.
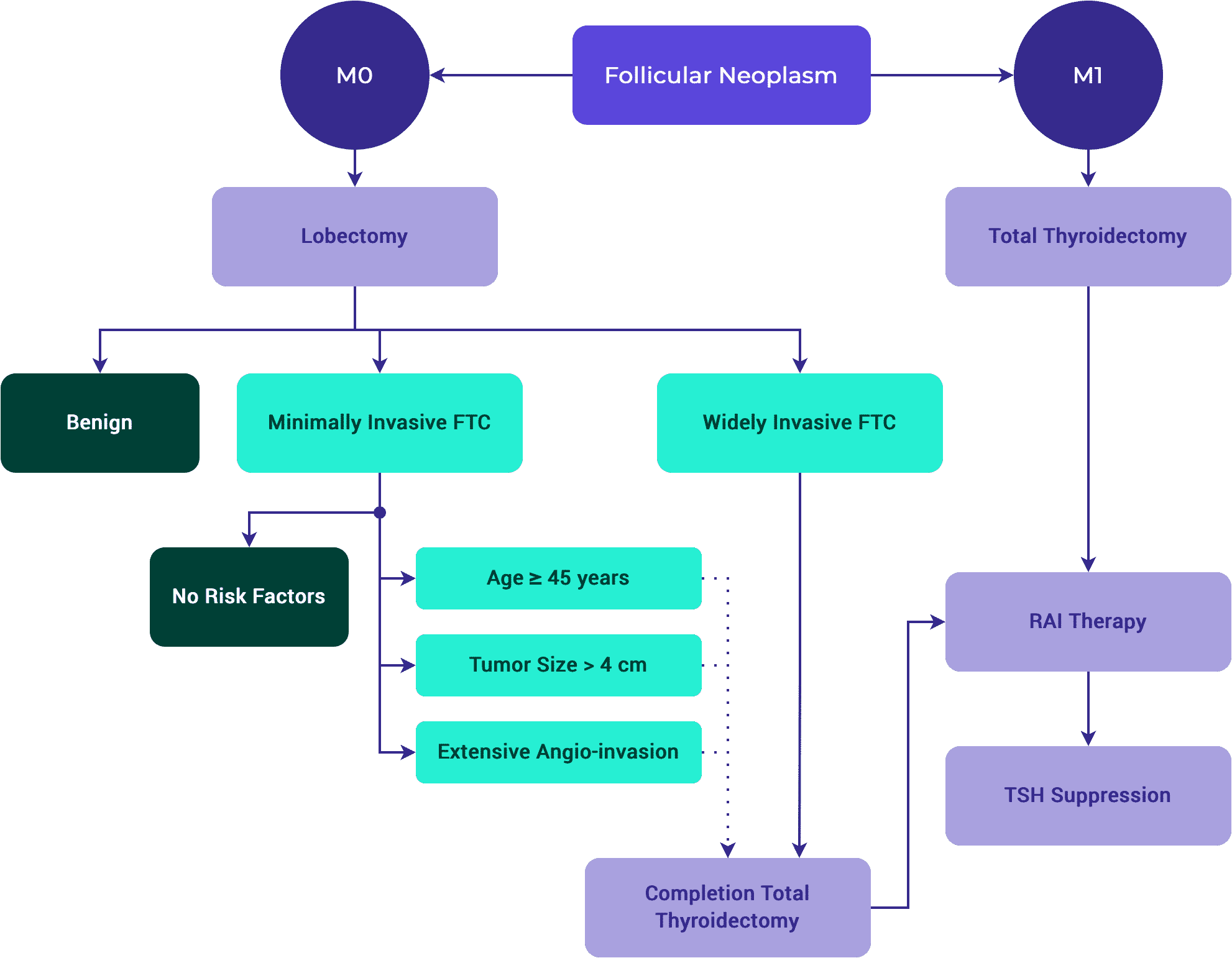
Management of Thyroid Cancer Patients after Lobectomy
Presenter: Jennifer Perkins, MD
Summary
- 4:13 — Choosing Surgical Approach Considerations
Dr. Perkins provides an overview of clinical factors that impact the decision of choosing between lobectomy versus total thyroidectomy. - 7:25 — Rising Thyroglobulin Level
Dr. Perkins presents a case of a 68-year-old female with left thyroid nodule noted incidentally on a carotid ultrasound. This patient underwent a left hemi-thyroidectomy and presented with rising thyroglobulin levels five years after surgery. Dr. Perkins presents other studies to examine whether thyroglobulin post-lobectomy is reliable. - 16:30 — Completion Thyroidectomy
Dr. Perkins presents a case of a 35-year-old female with palpable thyroid nodule noted on physical examination. FNA revealed a 1.9 cm papillary thyroid cancer, tall cell variant. Dr. Perkins evaluates whether this patient should receive completion thyroidectomy, and if so, whether this patient should receive radioactive iodine ablation. - 23:36 — Vascular Invasion
Dr. Perkins shares a case of a 54-year-old woman who underwent a right lobectomy. As this patient presented with two vessels of vascular invasion, Dr. Perkins examines the impact of vascular invasion and capsular invasion. - 36:26 — Discussion with Q&A

Add Upcoming TIROxMDS Seminars to Your Calendar
In 2 quick steps, you can add our entire schedule of upcoming seminars to your calendar today! Then you will get notified of upcoming lectures, presentations and case-based studies. Every Friday at 8:00 AM EST, we cover a new topic from published research.
Add to CalendarReferences
- 1.Haugen B, Alexander E, Bible K, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. doi:10.1089/thy.2015.0020
- 2.Patel K, Yip L, Lubitz C, et al. Executive Summary of the American Association of Endocrine Surgeons Guidelines for the Definitive Surgical Management of Thyroid Disease in Adults. Ann Surg. 2020;271(3):399-410. doi:10.1097/SLA.0000000000003735
- 3.Haddad RI, Nasr C, Bischoff L, et al. NCCN Guidelines Insights: Thyroid Carcinoma, Version 2.2018. J Natl Compr Canc Netw. Published online December 2018:1429-1440. doi:10.6004/jnccn.2018.0089
- 4.Ito Y, Onoda N, Okamoto T. The revised clinical practice guidelines on the management of thyroid tumors by the Japan Associations of Endocrine Surgeons: Core questions and recommendations for treatments of thyroid cancer. Endocr J. 2020;67(7):669-717. doi:10.1507/endocrj.EJ20-0025
- 5.Filetti S, Durante C, Hartl D, et al. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30(12):1856-1883. doi:10.1093/annonc/mdz400